Spevigo (spesolimab) has a conditional marketing authorisation for the treatment of generalised pustular psoriasis (GPP) flares in adults and adolescents from 12 years of age as monotherapy
▼This medicinal product is subject to additional monitoring. * Additional efficacy and safety data are being collected.
Click here for prescribing information.
Resources for you

SPEVIGO®▼ (spesolimab) booklet

SPEVIGO®▼ (spesolimab) dosing and administration guide

GPP Medical Education website
This website is for UK Healthcare Professionals. Patients should be directed to the GPP HUB patient website and not the UK Healthcare Professional site
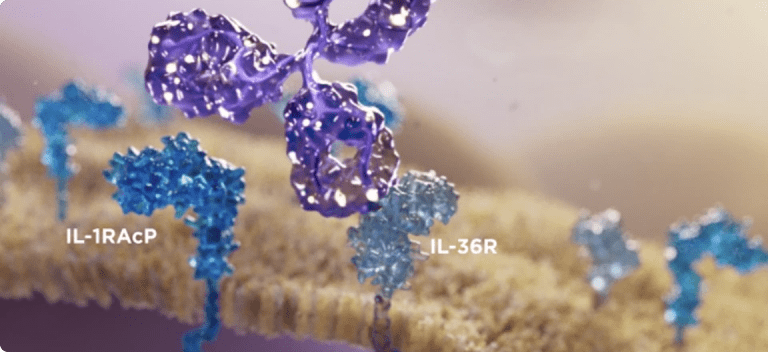
See how SPEVIGO®▼ (spesolimab) works
Watch now
Resources for your patients

GPP information brochure
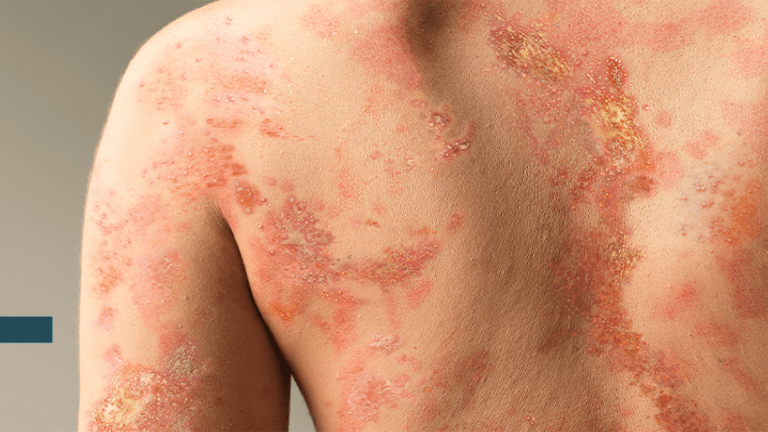
GPP HUB
Patients should be directed to the GPP HUB patient website and not the UK Healthcare Professional site

Living well with GPP: a podcast series
PC-GB-109369 V2 | March 2025

