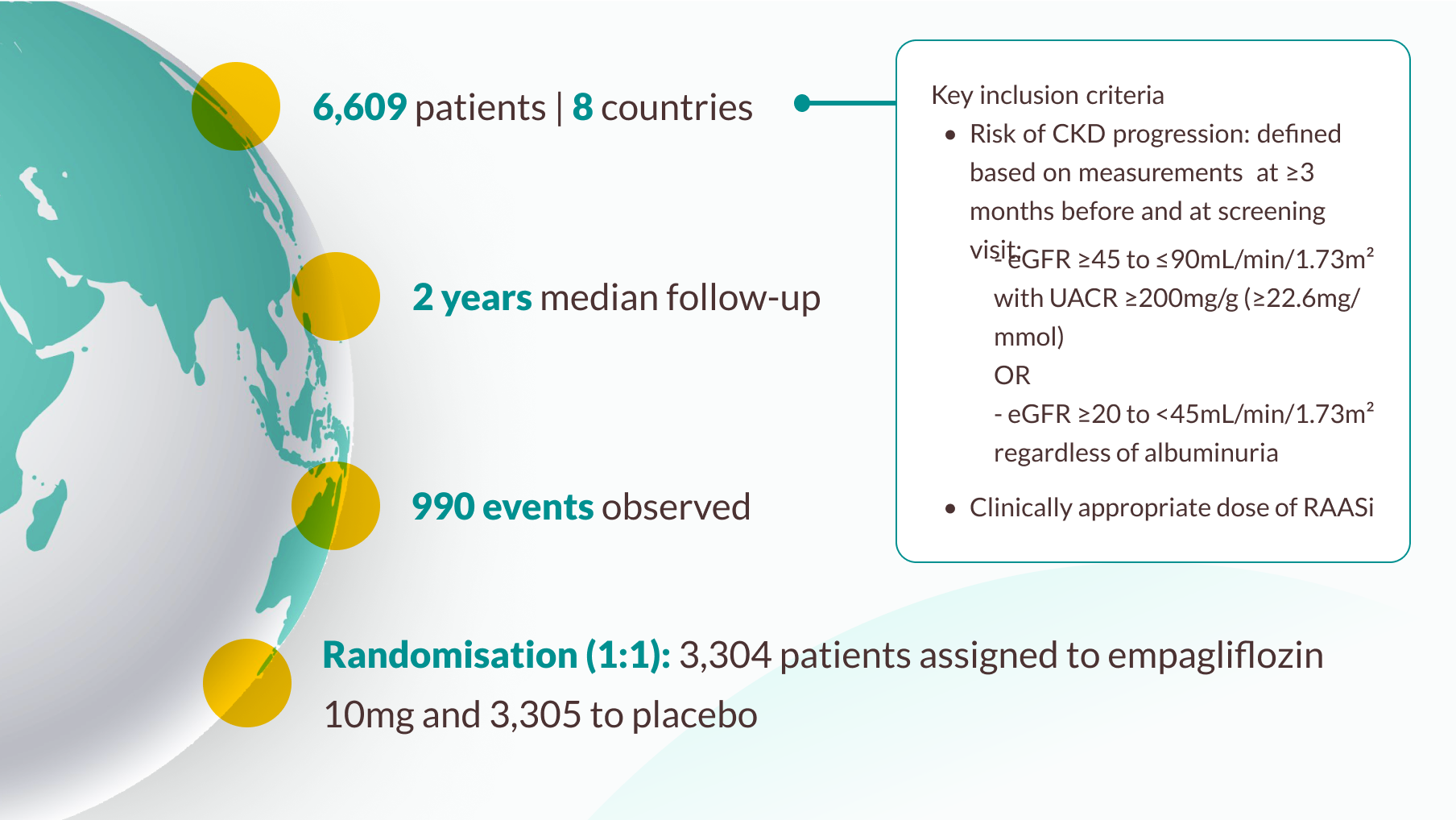
Clinical trial

Efficacy results
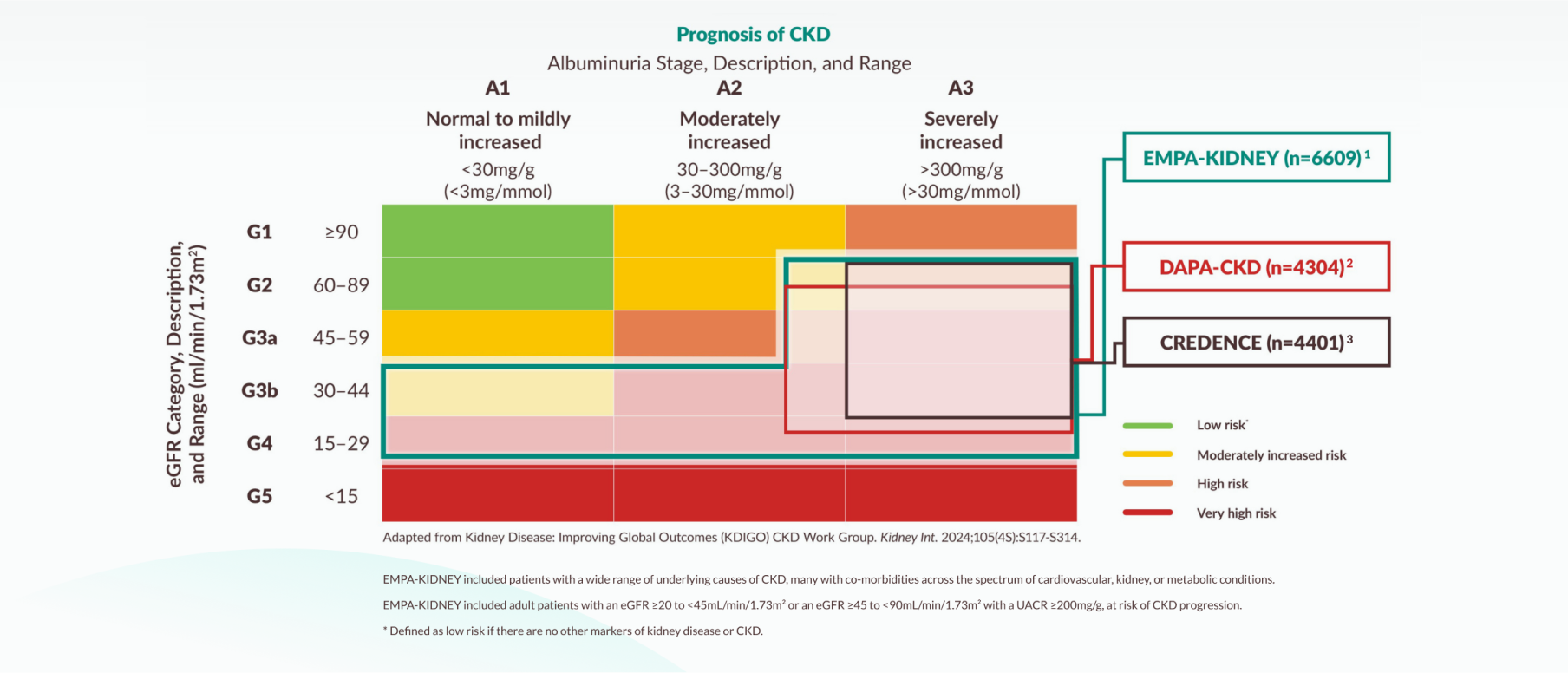
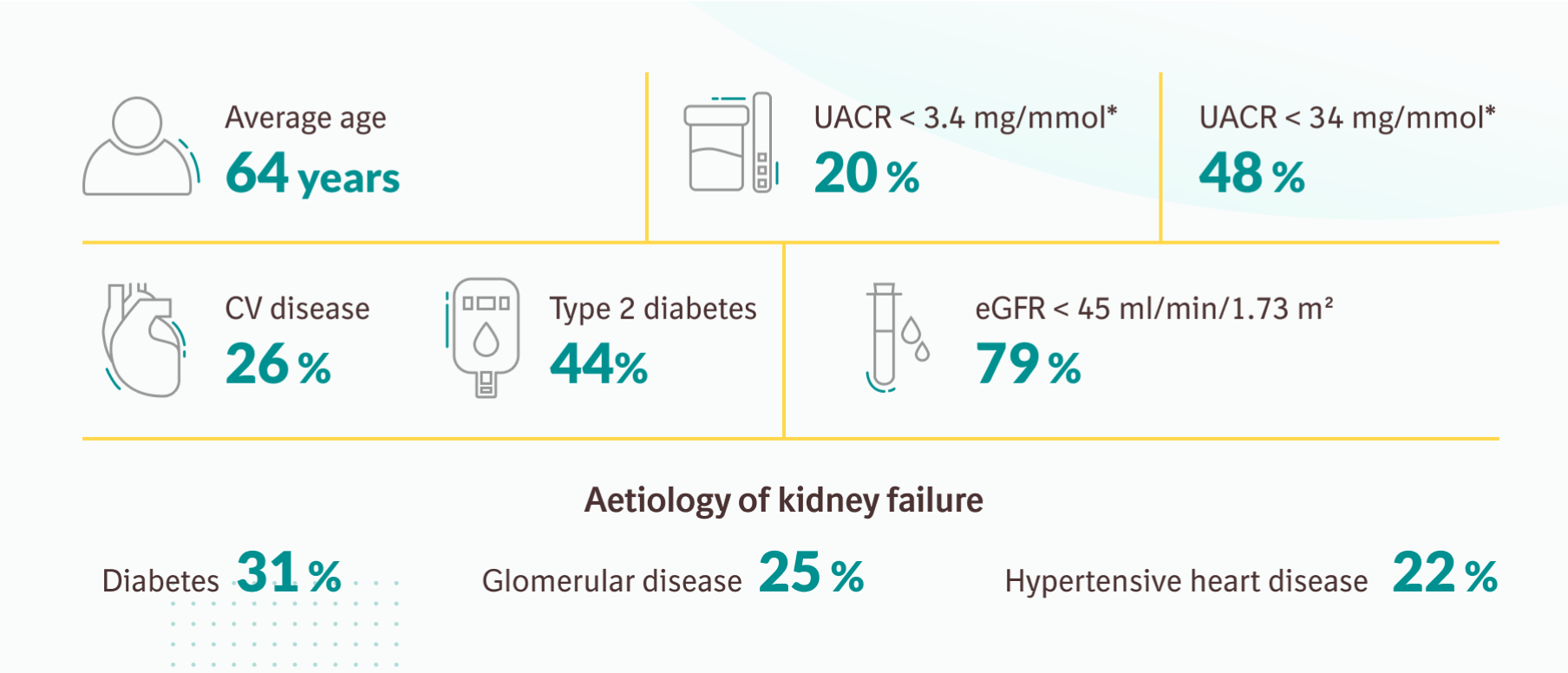
Efficacy results: EMPA-KIDNEY trial
Primary outcome of EMPA-KIDNEY
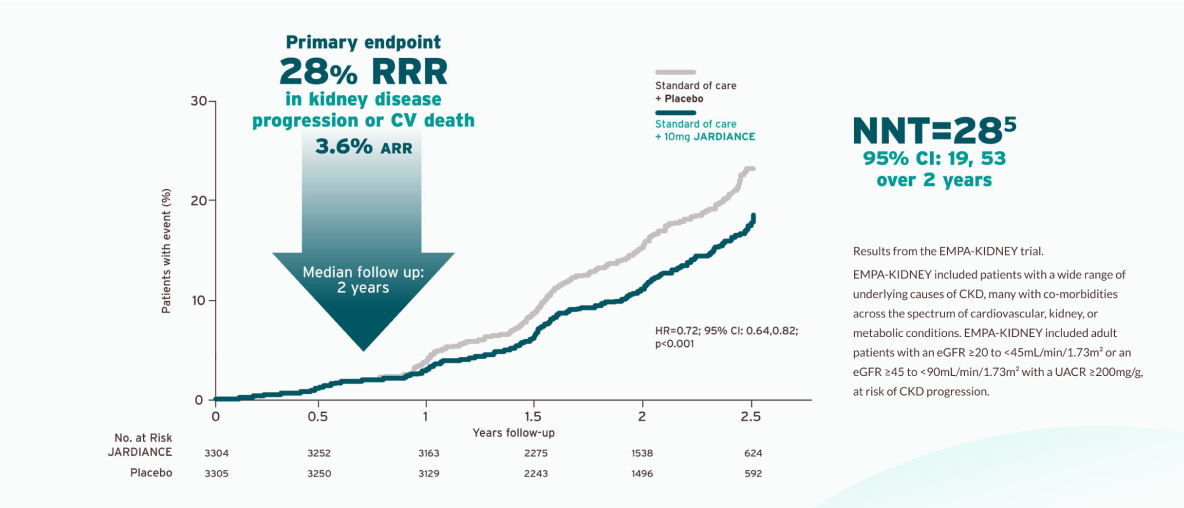
JARDIANCE® reduced risk of kidney disease progression or CV death vs placebo1,4
In EMPA-KIDNEY, empagliflozin reduced the risk of kidney disease progression or cardiovascular death by 28% (3.6 ARR) compared to placebo (HR 0.72, 95% CI 0.64–0.82; p<0.001). Over a median follow-up of 2 years, events occurred in 13.1% of patients in the empagliflozin group and 16.9% in the placebo group, including those with an eGFR range from 20 to <45 mL/min/1.73 m2 and those with eGFR ≥45 to <90 mL/min/1.73 m2 with albuminuria.
Secondary outcome of EMPA-KIDNEY
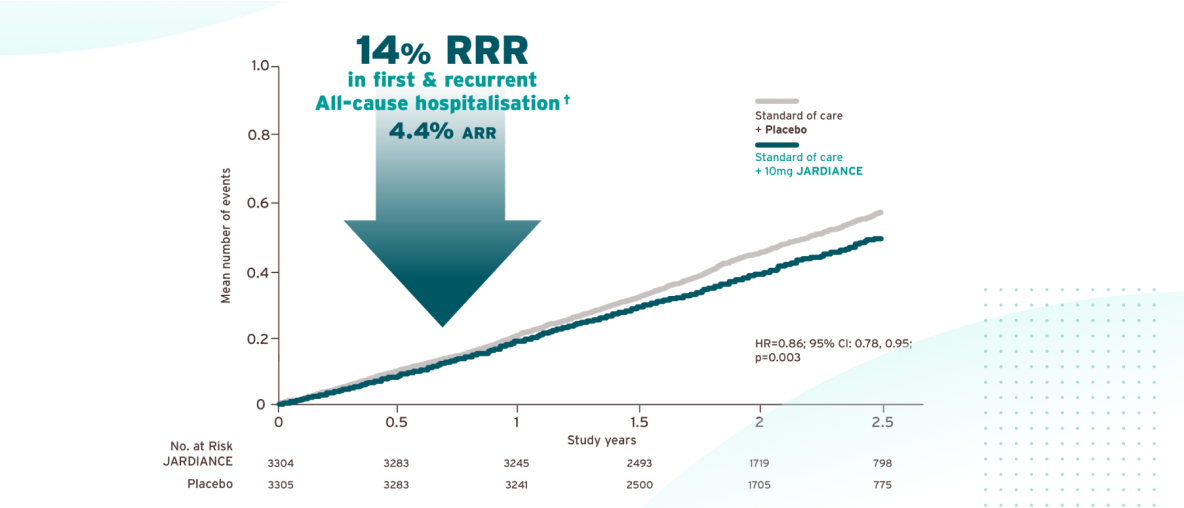
JARDIANCE® helped protect patients by keeping them out of hospital vs placebo.1,5
In EMPA-KIDNEY, empagliflozin reduced the risk of first and recurrent all-cause hospitalisations by 14% compared to placebo (HR 0.86, 95% CI 0.78–0.95; p=0.003), resulting in a 4.4% absolute risk reduction (ARR) over a median follow-up of 2 years. This reduction was observed consistently across patient subgroups, including those with varying degrees of eGFR and albuminuria. Empagliflozin demonstrated a impact on reducing the burden of hospitalisations in patients with chronic kidney disease across a broad range of underlying cardiovascular, kidney, or metabolic conditions.
Exploratory outcome of EMPA-KIDNEY
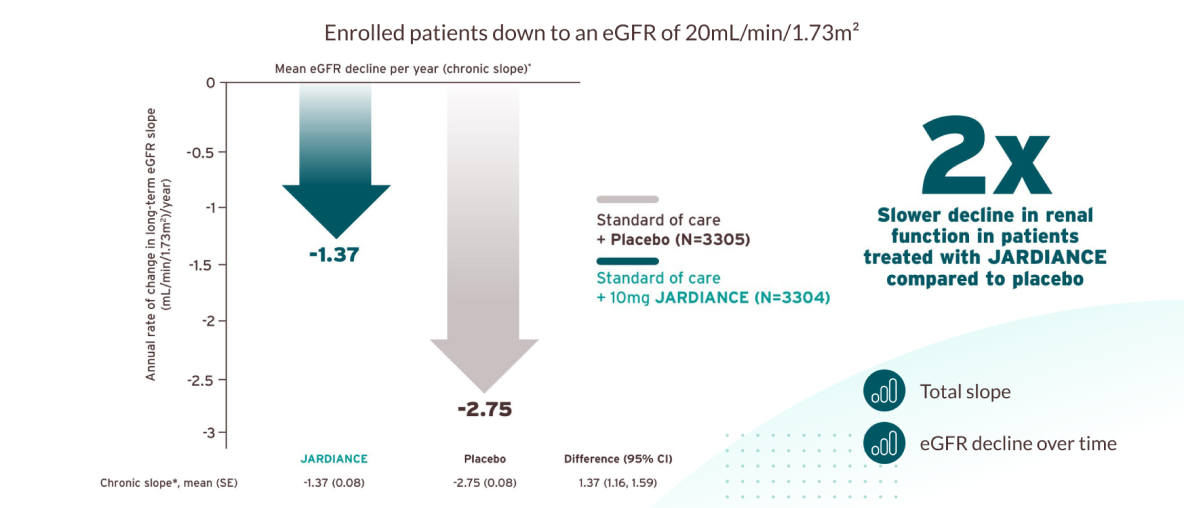
JARDIANCE® protected the kidneys by slowing the decline of renal function over time and could delay time to dialysis.1
In EMPA-KIDNEY, empagliflozin slowed the decline in renal function (chronic slope), with a mean annual eGFR decline of -1.37 mL/min/1.73 m2 per year, compared to -2.75 mL/min/1.73 m2 per year in the placebo group. This suggests that empagliflozin may delay the progression to dialysis or advanced kidney disease in patients with CKD, potentially offering long-term renal benefits.
Exploratory outcome: (Chronic slope, referred to as ‘’Long term’’): Mean annual rates of change in eGFR from 2 months to the final follow-up visit by treatment allocation were estimated using shared parameter models.
Exploratory outcome of EMPA-KIDNEY
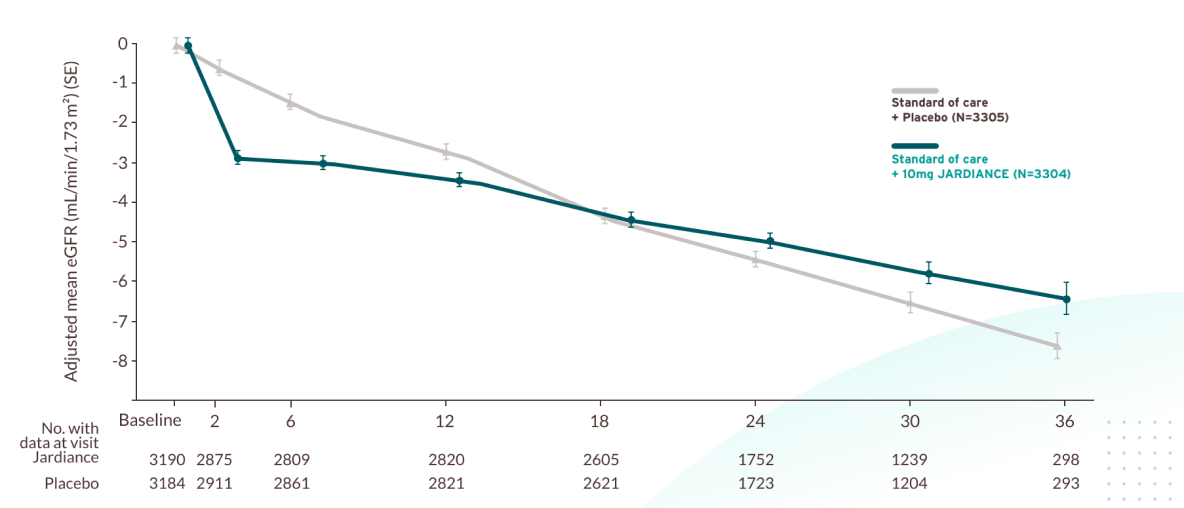
Annual rate of change in eGFR1
Empagliflozin slowed the decline in renal function (total slope) by -2.16 mL/ min/1.73 m2 per year compared to -2.92 mL/min/1.73 m2 per year in the placebo group. This endpoint was not tested for statistical significance due to its position within the testing hierarchy.
Exploratory outcome included the mean annual rates of change in eGFR in ml/ min/1.73m2 per year from baseline to the final follow-up visit (“total slopes”) by treatment allocation were estimated using shared parameter models.
Prespecified exploratory outcome from EMPA-KIDNEY
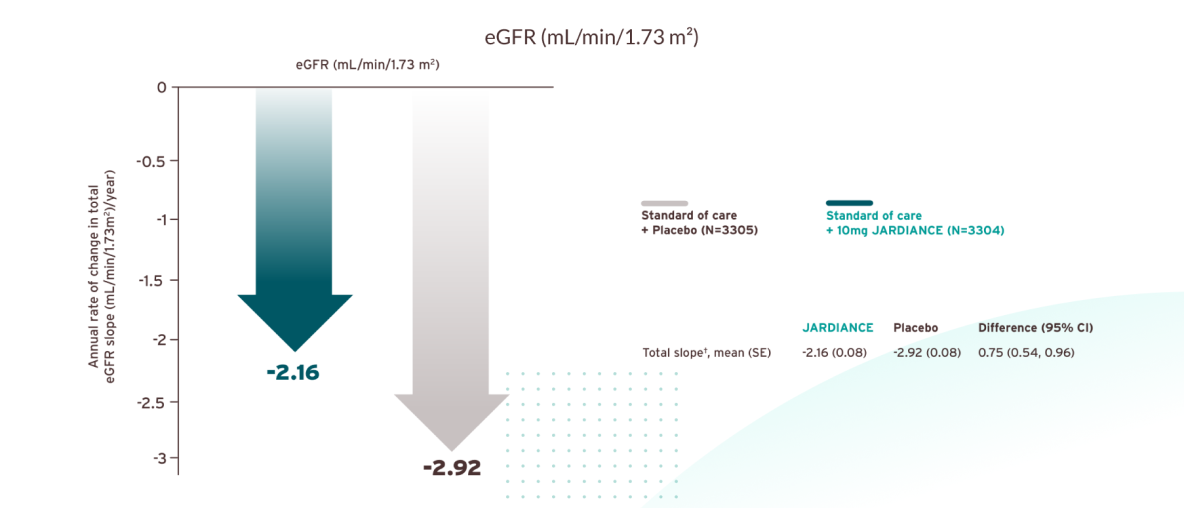
JARDIANCE® slowed the decline of kidney function vs placebo1,6
An initial reduction in eGFR was observed with empagliflozin, which stabilised over time, resulting in a total mean change of the total slope by -2.16 mL/ min/1.73 m2 per year with empagliflozin, compared to -2.92 mL/min/1.73 m2 per year with placebo (difference: 0.75 mL/min/1.73 m2; 95% CI, 0.54–0.96). These results indicate a slower progression of chronic kidney disease in patients treated with empagliflozin. This endpoint was not tested for statistical significance due to its position within the testing hierarchy.
Prespecified exploratory outcome. Linear mixed models for repeated measures analyses were used to estimate the mean estimated GFR in each group at each scheduled follow-up visit
Want to discuss further?
Knowledge base
JARDIANCE® is simple to initiate at 10 mg dosing across all indications.

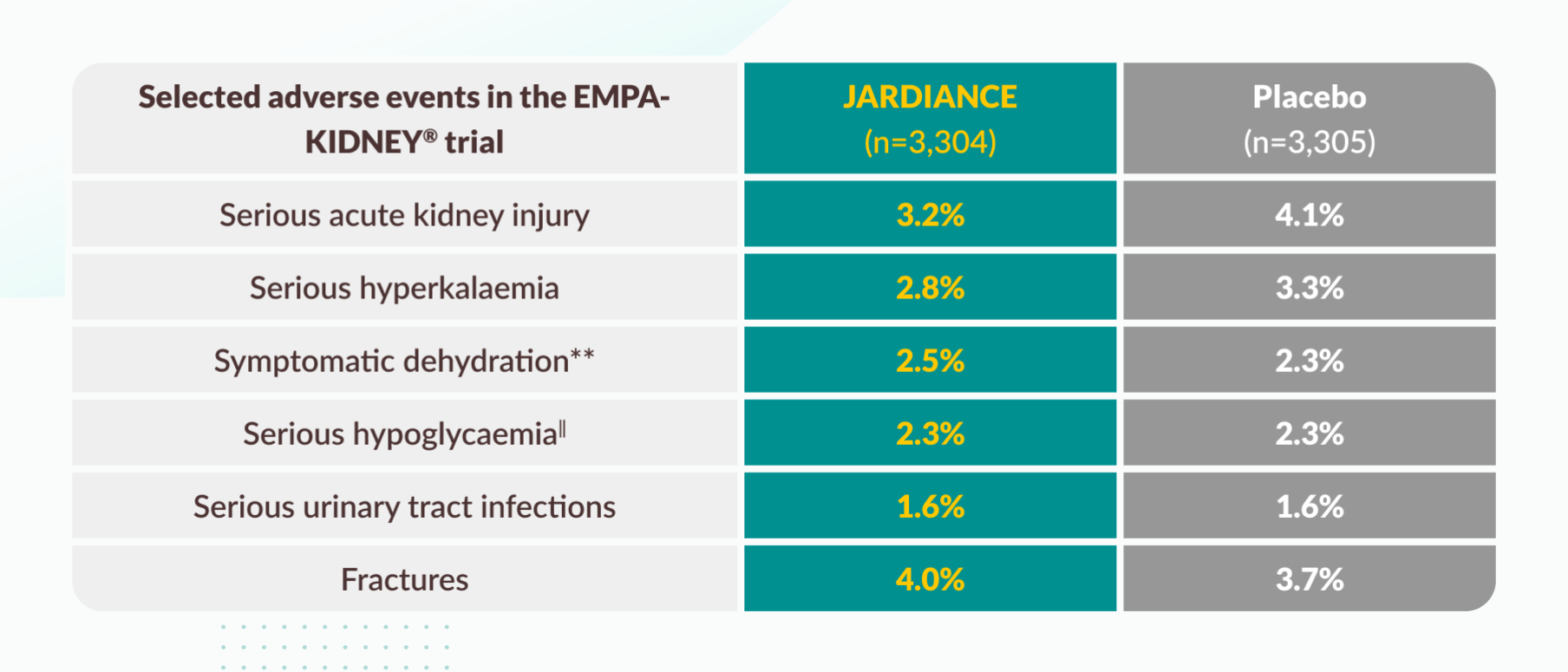
Adverse reactions from reported placebo-controlled studies and post-marketing experience.

Abbreviations
ARR: absolute risk reduction; CI: confidence interval; CKD: chronic kidney disease; CV: cardiovascular; eGFR: estimated glomerular filtration rate; HR: hazard ratio; RAASi: renin-angiotensin-aldosterone system inhibitor; RRR: relative risk reduction; SE: standard error of the mean; SGLT2: sodium-glucose cotransporter-2; T2D: type 2 diabetes; UACR: urinary albumin-to-creatinine ratio.
- Herrington WG, et al. N Engl J Med. 2023;388(2):117-127. (EMPA-KIDNEY results and the publication’s Supplementary Appendix).
- Heerspink H, et al. N Engl J Med. 2020;383:1436-1446.
- Perkovic V, et al. N Engl J Med. 2019;380:2295-2306.
- JARDIANCE Data on File (EMP 23-22).
- JARDIANCE Data on File (EMP 23-13).
- JARDIANCE Data on File (EMP 23-20).
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. Kidney Int. 2024;105(4S):S117–S314.
PC-GB-110620 | December 2024
The content on this website is in relation to adult patients.
Empagliflozin is not recommended in severe hepatic impairment, breastfeeding, Type 1 diabetes and is contraindicated in patients with hypersensitivity to the active ingredient or any of its excipients. Empagliflozin should be avoided in pregnancy.
Please consult the SmPC for full details regarding adverse events, monitoring requirements and interactions prior to prescribing JARDIANCE®.
- JARDIANCE® (empagliflozin) UK Summary of Product Characteristics (SmPC). Available at:
http://www.medicines.org.uk/emc/medicine/28973.
JARDIANCE® is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise
- as monotherapy when metformin is considered inappropriate due to intolerance
- in addition to other medicinal products for the treatment of diabetes1
JARDIANCE® is indicated in adults for the treatment of chronic kidney disease.1
JARDIANCE® is indicated in adults for the treatment of symptomatic chronic heart failure.1
PC-GB-109995 V4 | October 2025