
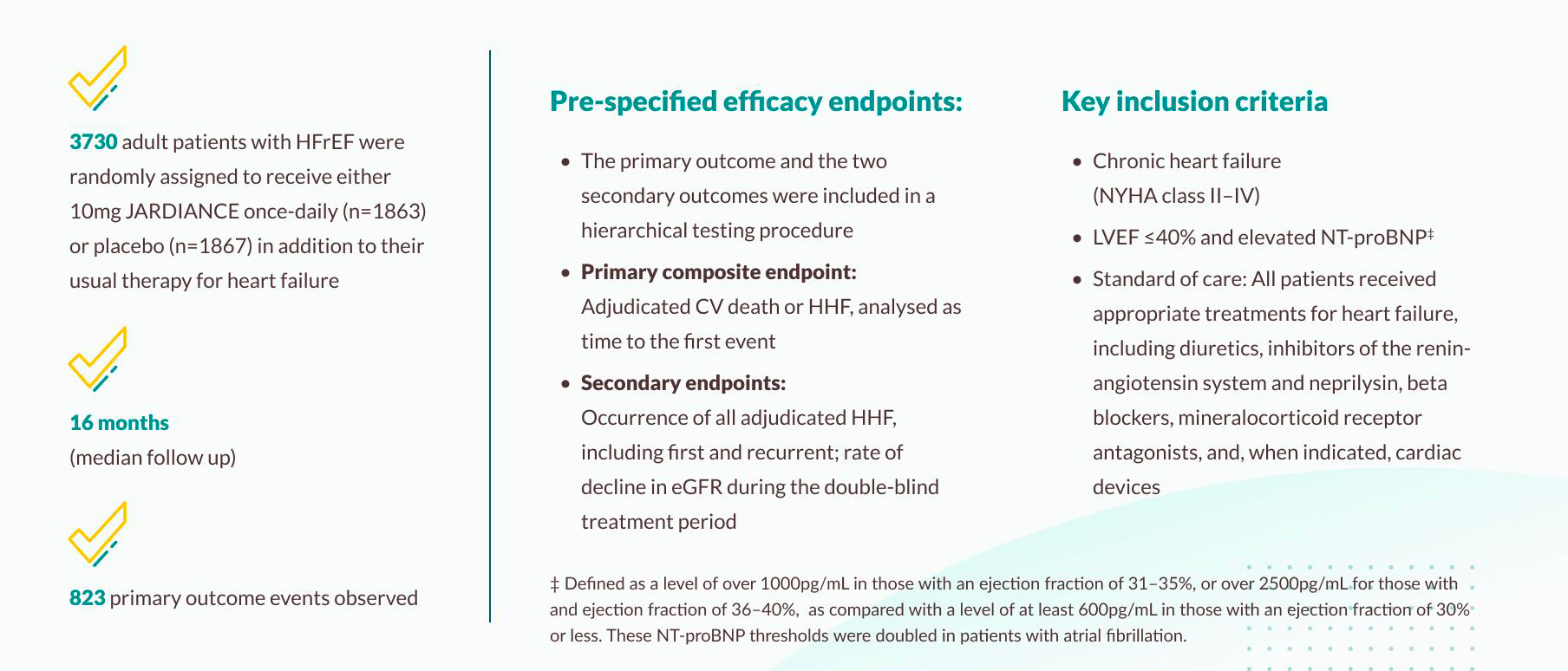
EMPEROR-Reduced trial

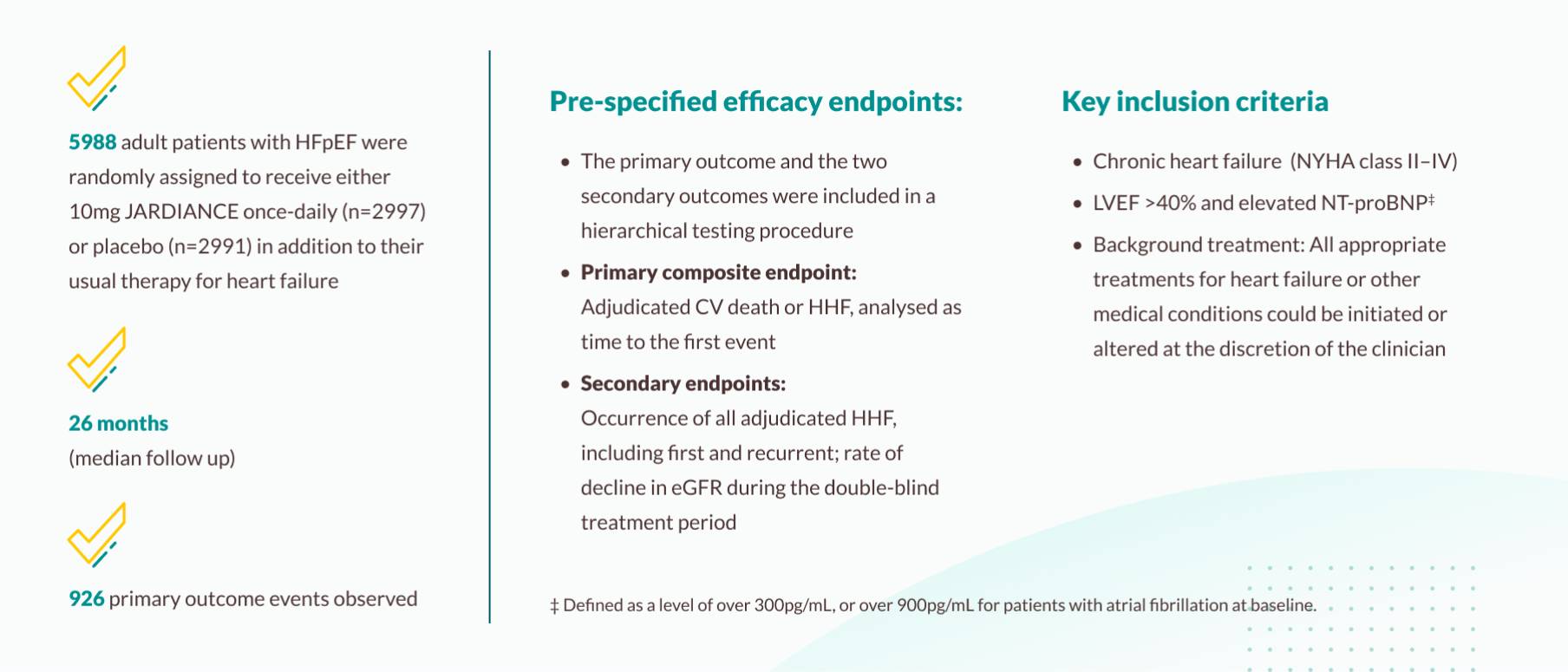
EMPEROR-Preserved trial
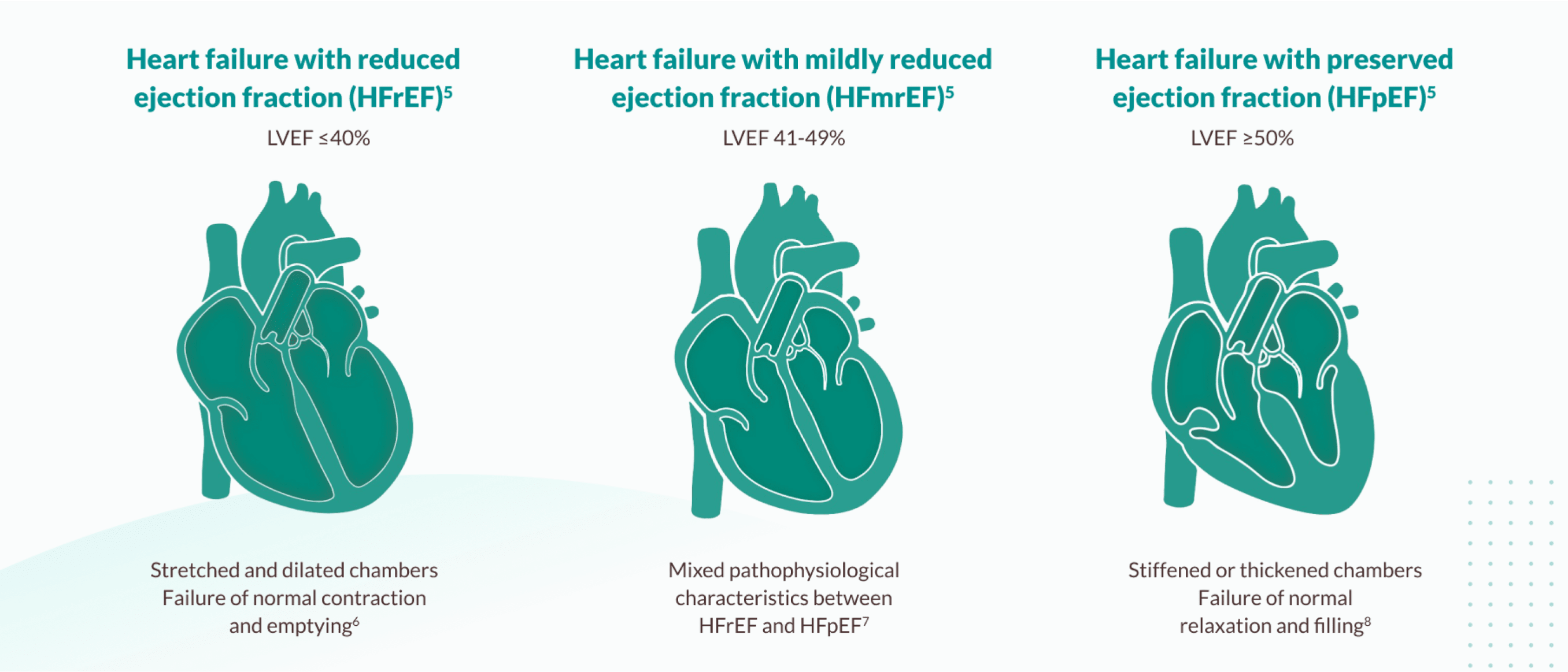
The ESC defined types of heart failure
Each type has different characteristics and is managed differently5
EMPEROR-Reduced trial1
The EMPEROR-Reduced trial investigated the efficacy of empagliflozin 10 mg versus placebo, in addition to guideline-directed medical therapy (GDMT), in patients with symptomatic chronic heart failure with reduced ejection fraction (LVEF ≤40%).
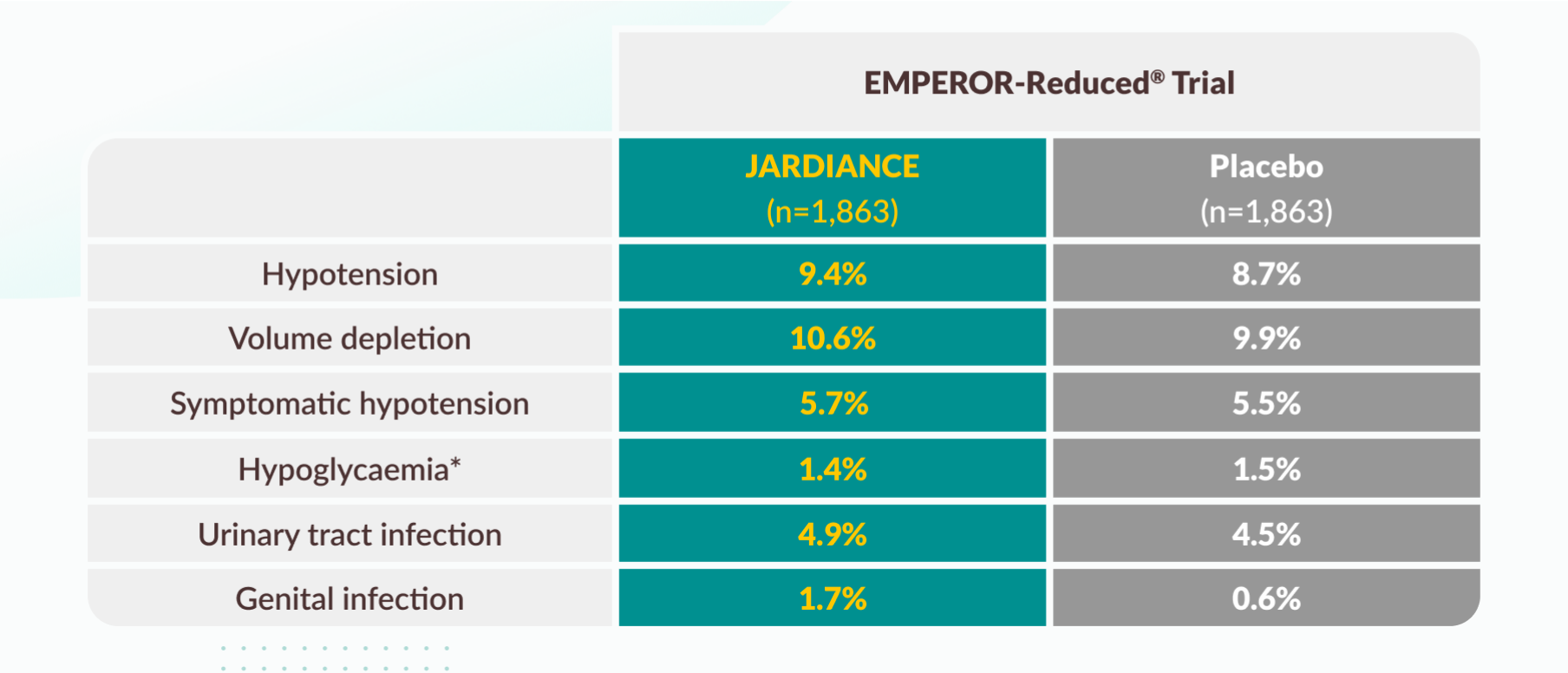
Tolerability profile
Selected adverse events in the EMPEROR-Reduced® trial.
Hide
* Hypoglycemic AEs with a plasma glucose value of ≤70 mg/dL or that required assistance.
Efficacy results: EMPEROR-Reduced trial
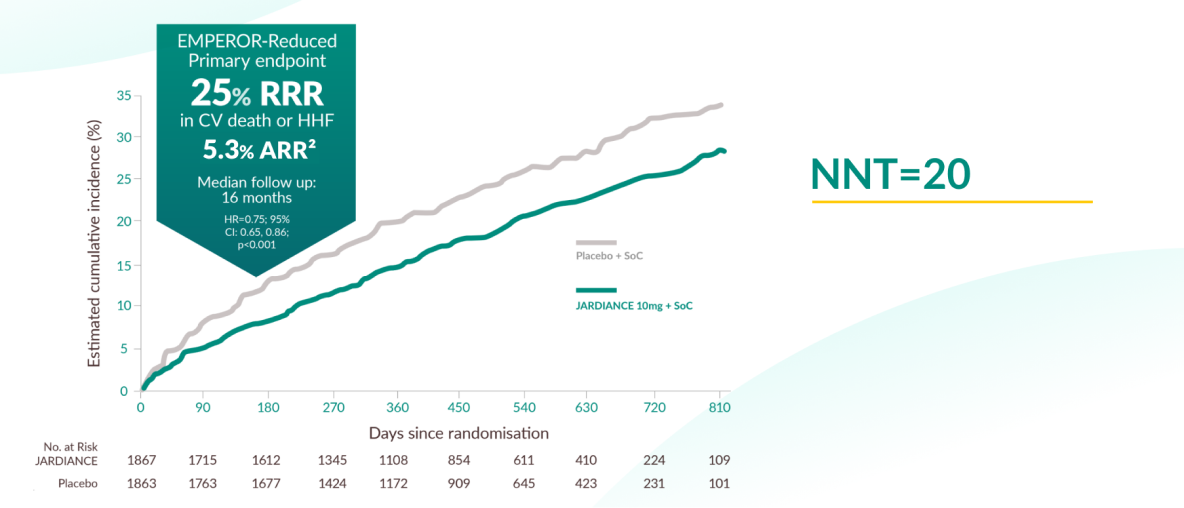
Primary outcome of the EMPEROR-Reduced trial
JARDIANCE® demonstrated superiority in reducing the risk of CV death or HHF in HF patients with LVEF ≤40% on top of SoC vs placebo1,11
The EMPEROR-Reduced trial demonstrated that treatment with JARDIANCE® (empagliflozin) reduced the risk of cardiovascular (CV) death or hospitalization for heart failure (HHF) compared to placebo in patients with heart failure and reduced ejection fraction (LVEF ≤40%). The primary composite endpoint showed a 25% relative risk reduction (RRR) and a 5.3% absolute risk reduction (ARR) in CV death or HHF (HR: 0.75; 95% CI: 0.65–0.86; p<0.001). The number needed to treat (NNT) to prevent one primary event over a 16-month period was 20 (95% CI: 13, 37).
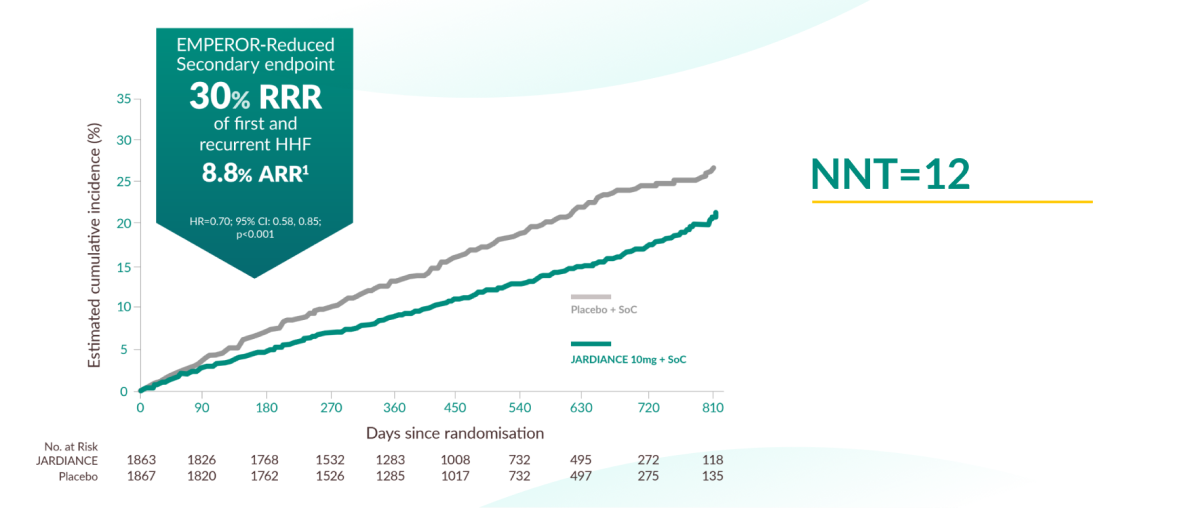
Secondary outcome of the EMPEROR-Reduced trial
JARDIANCE® reduced total hospital admissions for heart failure in HF patients with LVEF ≤40% on top of SoC vs placebo1,11
The total number of hospitalisations for heart failure was lower in the JARDIANCE® group than in the placebo group, with 388 events vs 553 events, respectively HR, 0.70; 95% CI, 0.58 to 0.85; p<0.001.
Based on the 30% RRR (8.8% ARR) demonstrated for hospitalisations for heart failure, 12 patients would need to have been treated with JARDIANCE® to prevent one event.
EMPEROR-Preserved trial2
The EMPEROR-Preserved trial investigated the efficacy of empagliflozin 10 mg versus placebo, in addition to background treatment, in patients with symptomatic chronic heart failure with preserved ejection fraction (LVEF >40%).
Tolerability profile
Selected adverse events in the EMPEROR-Preserved® trial.
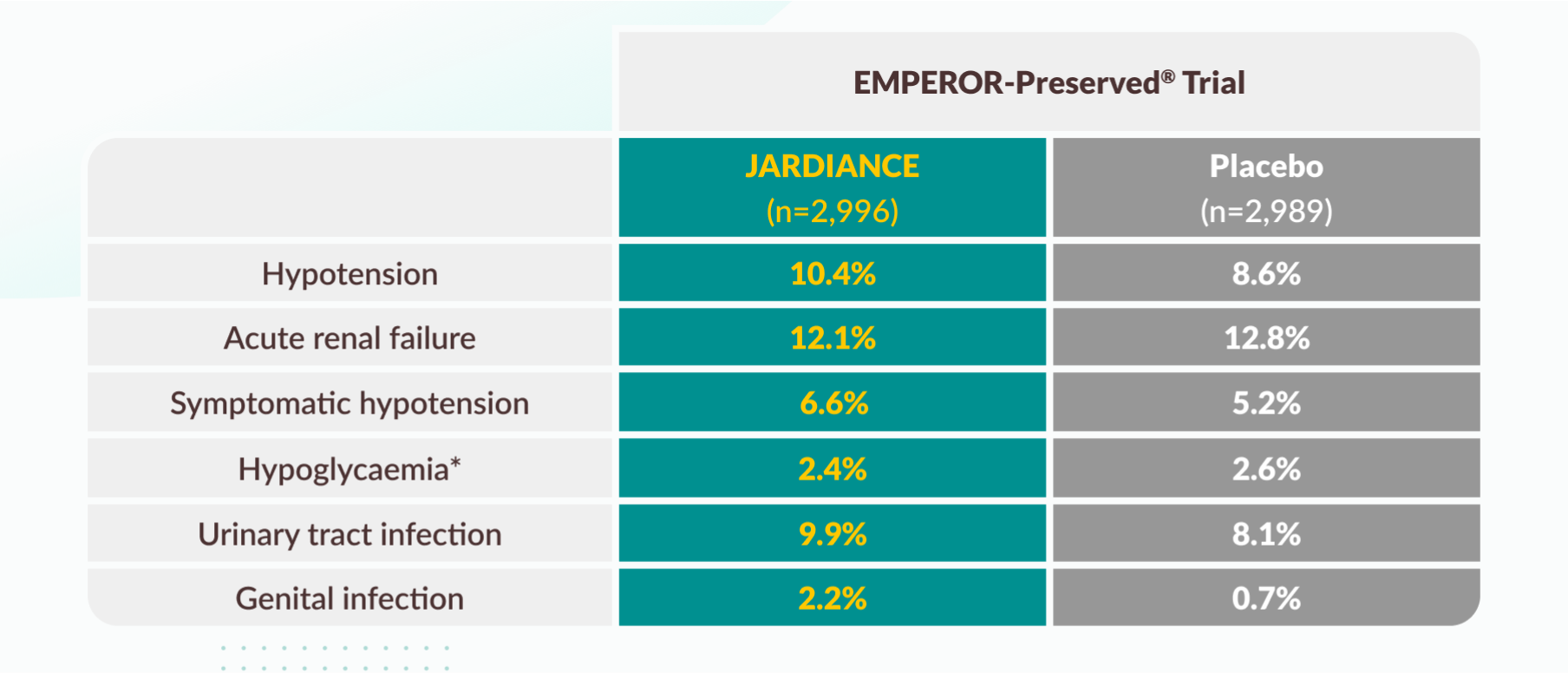
* Hypoglycemic AEs with a plasma glucose value of ≤70 mg/dL or that required assistance.
Efficacy results: EMPEROR-Preserved trial
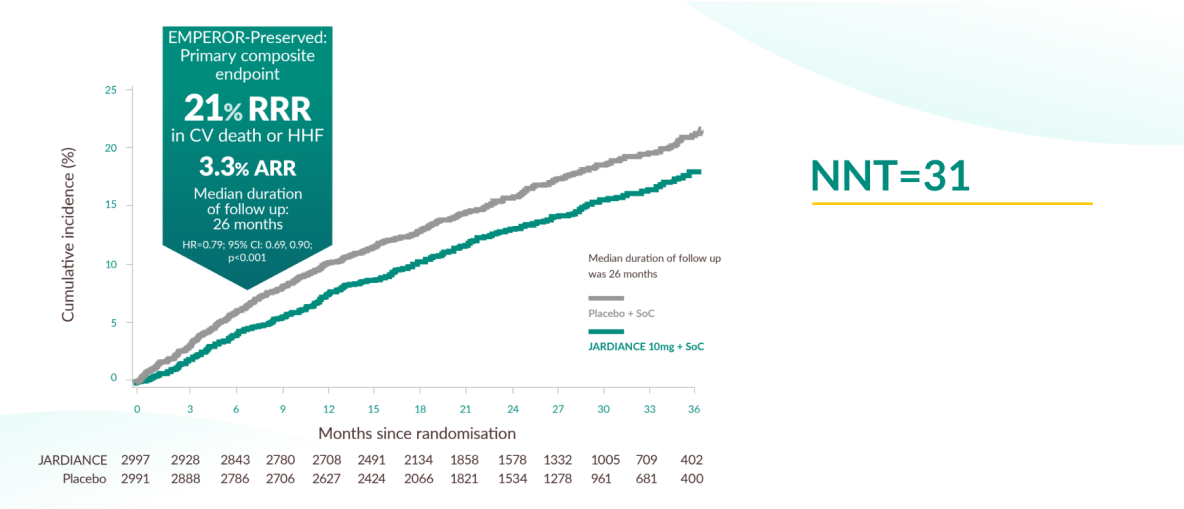
Primary outcome of EMPEROR-Preserved
JARDIANCE® demonstrated superiority in reducing the risk of CV death or HHF in HF patients with LVEF >40% on top of SoC vs placebo1,11
Subgroup analysis showed efficacy was consistent across the spectrum of LVEF.14 The primary composite outcome occurred in 415 patients (13.8%) in the empagliflozin group and 511 patients (17.1%) in the placebo group (HR 0.79; 95% CI, 0.69–0.90; p<0.001).
With a 21% relative risk reduction (3.3% absolute risk reduction) in CV death or hospitalisation for heart failure (HHF), the number needed to treat (NNT) to prevent one primary event was 31 (95% CI, 20–69).
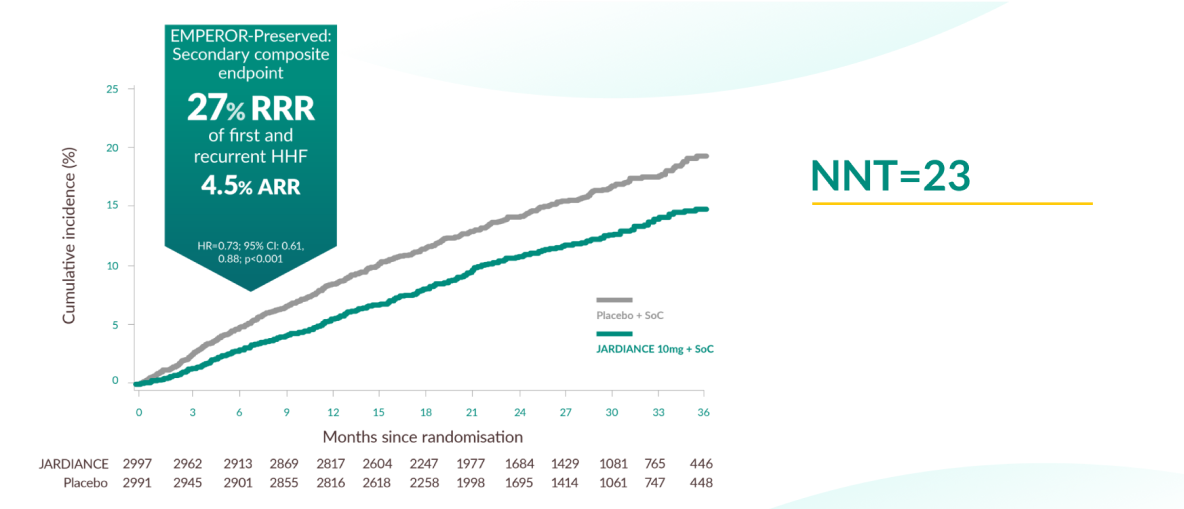
Secondary composite outcome of EMPEROR-Preserved
JARDIANCE® demonstrated superiority in reducing the risk of first or recurrent HHF in HF patients with LVEF >40% vs placebo.2
The total number of HHF was lower in the JARDIANCE® group than in the placebo group, with 407 events vs 541 events, respectively (HR, 0.73; 95% CI, 0.61 to 0.88; p<0.001).
Based on the 27% RRR (4.5% ARR) demonstrated for HHF, 22 patients would need to have been treated with JARDIANCE® to prevent one event.
Want to discuss further?
Knowledge base
JARDIANCE® is simple to initiate at 10 mg dosing across all indications.

Adverse reactions from reported placebo-controlled studies and post-marketing experience.

Abbreviations
3P-MACE: 3-point major adverse cardiovascular events; AE: adverse event; ARR: absolute risk reduction; CHF: chronic heart failure; CI: confidence interval; CV: cardiovascular; HHF: hospitalisation due to heart failure; CVD: cardiovascular disease; DPP-4: dipeptidyl-peptidase 4; EASD: European Association for the Study of Diabetes; eGFR: estimated glomerular filtration rate; ESC: European Society of Cardiology; HF: heart failure; HFmrEF: heart failure with mildly reduced ejection fraction; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; HHF: hospitalisation for heart failure; HR: hazard ratio; KCCQ: Kansas City Cardiomyopathy Questionnaire; LVEF: left ventricular ejection fraction; MACE: major adverse cardiac events; MI: myocardial infarction; NEJM: New England Journal of Medicine; NT-proBNP: N-terminal pro hormone B-type natriuretic peptide; NYHA: New York Heart Association; RRR: relative risk reduction; T2D: type 2 diabetes.
- Packer M, et al. N Engl J Med. 2020;383(15):1413-1424. (& Supplementary appendix).
- Anker SD, et al. N Engl J Med. 2021;385(16):1451–1461.
- Butler J, et al. Eur Heart J. 2021;42:1203–1212.
- Butler J, et al. Circulation. 2022;145:184–193.
- McDonagh TA, et al. Eur Heart J. 2021;42(36):3599–3726.
- Kemp CD, Conte JV. Cardiovasc Pathol. 2012;21:365–371.
- Zhou Q, et al. Front Cardiovasc Med. 2021;8:678121.
- Obokata M, et al. JACC Cardiovasc Imaging. 2020;13(1 Pt 2):245–257.
- Bohm M, et al. J Am Coll Cardiol. 2022;80:1–18.
- Zinman B, et al. N Engl J Med. 2015;373:2117–2128.
- JARDIANCE® (empagliflozin) Summary of Product Characteristics (SmPC). Available at: http://www.medicines.org.uk/emc/medicine/28973.
- Packer M et al. Circulation 2021;143(4)326–336 and supplementary appendix.
- Butler J, et al. Eur J Heart Failure. 2022;24:245–248.
- Anker SD, et al. Nat Med. 2022;28:2512–2520.
PC-GB-110621 | December 2024
The content on this website is in relation to adult patients.
Empagliflozin is not recommended in severe hepatic impairment, breastfeeding, Type 1 diabetes and is contraindicated in patients with hypersensitivity to the active ingredient or any of its excipients. Empagliflozin should be avoided in pregnancy.
Please consult the SmPC for full details regarding adverse events, monitoring requirements and interactions prior to prescribing JARDIANCE®.
- JARDIANCE® (empagliflozin) UK Summary of Product Characteristics (SmPC). Available at:
http://www.medicines.org.uk/emc/medicine/28973.
JARDIANCE® is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise
- as monotherapy when metformin is considered inappropriate due to intolerance
- in addition to other medicinal products for the treatment of diabetes1
JARDIANCE® is indicated in adults for the treatment of chronic kidney disease.1
JARDIANCE® is indicated in adults for the treatment of symptomatic chronic heart failure.1
PC-GB-109995 V4 | October 2025

