How would you treat a severe asthma patient?
SPIRIVA® Respimat® (tiotropium) is indicated as add-on maintenance bronchodilator treatment in patients aged 6 years and older with severe asthma who experienced one or more severe asthma exacerbations in the preceding year. In adult patients with severe asthma, tiotropium should be used in addition to inhaled corticosteroids (≥800 μg budesonide/day or equivalent) and at least one controller.
About Severe Asthma
Severe asthma is a specific type of difficult-to-treat asthma in which patients struggle to control their symptoms and may experience frequent exacerbations despite maximal optimised treatment and effectively managed co-morbidities and contributory factors (such as therapy adherence and inhaler technique).1,2
Uncontrolled, difficult-to-treat, and severe asthma2

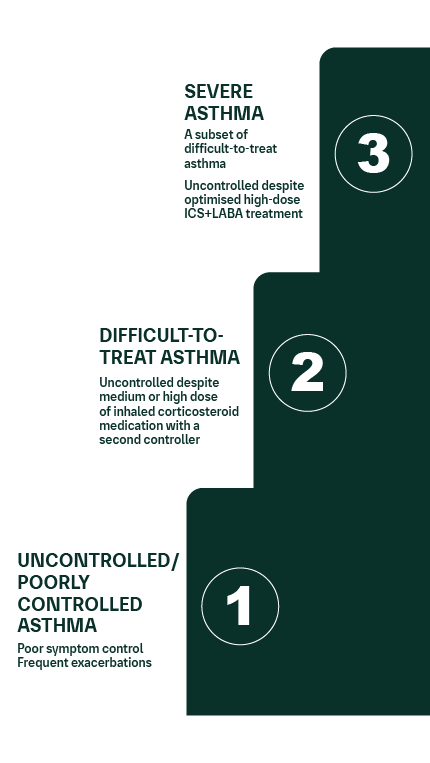
Severe asthma is associated with significant morbidity and mortality, as well as treatment, psychological, and socioeconomic burdens.2,3
What is the impact of severe asthma in the UK?
Up to 79%
~4%
~200,000
≥4x
The HASTE tool
The HASTE tool was developed by the Oxford Academic Health Science Network as an aide memoire for clinicians in primary care to help decide when to consider referral to secondary care. If asthma does not improve in response to optimising treatment and the answers to the HASTE questions are yes, then consider referring to a specialist in asthma care for further assessment.2,7
High-intensity treatment
Is the patient already at the high end of the treatment escalator?
Adherence
Are patients taking their medication at the correct dose and frequency?
Severe Exacerbations
Has the patient had at least 2 courses of oral corticosteroids or been hospitalised due to asthma in the last 12 months?
Technique
Is the patient’s inhaler technique correct?
Exclude other conditions
Are conditions that mimic or exacerbate asthma being managed?
Abbreviations:
BTS, British Thoracic Society; GINA, Global Initiative for Asthma; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonist; NICE, National Institute for Health and Care Excellence; MART, maintenance and reliever therapy; SIGN, Scottish Intercollegiate Guidelines Network.
References
- Asthma UK. Slipping through the net: The reality facing patients with difficult and severe asthma. Available at: https://www.asthmaandlung.org.uk/sites/default/files/2023-03/auk-severe-asthma-gh-final.pdf (accessed February 2025).
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention. Available at: https://ginasthma.org/reports/ (accessed February 2025).
- Wang E et al. Chest 2020;157(4):790–804
- Asthma UK. Asthma Care in a Crisis: Annual Asthma Survey 2020. Available at: https://www.asthmaandlung.org.uk/sites/default/files/2023-03/aas-2020_2a-1.pdf (accessed February 2025).
- Asthma + Lung UK. What is severe asthma? Available at: https://www.asthmaandlung.org.uk/conditions/severe-asthma/what-severe-asthma (accessed February 2025).
- Kerkhof M et al. Thorax 2018;73:116–124.
- NHS. Recognising uncontrolled asthma in primary care: the HASTE tool. Available at: https://www.healthinnovationoxford.org/wp-content/uploads/2022/05/AB-podcast-poster.pdf (Accessed February 2025)
PC-GB-109485 V1 February 2025


