Spevigo (spesolimab) has a conditional marketing authorisation for the treatment of generalised pustular psoriasis (GPP) flares in adults and adolescents from 12 years of age as monotherapy
▼This medicinal product is subject to additional monitoring. * Additional efficacy and safety data are being collected.
Click here for prescribing information.
GPP is a rare, debilitating, systemic skin disease with life-threatening potential1-4
GPP flares can erupt suddenly and escalate quickly, with the potential to lead to complications requiring emergency hospitalisation3-6
GPP flares:
Characterised by widespread eruption of pustules, erythema, and scaling6-8
Substantially impact patients’ quality of life, social relationships, and mental health2,3,6,9-11
Vary in symptom duration and severity5,6
May become life-threatening if left untreated3,4,6
A patient with a GPP flare from the Effisayil™ 1 clinical trial12
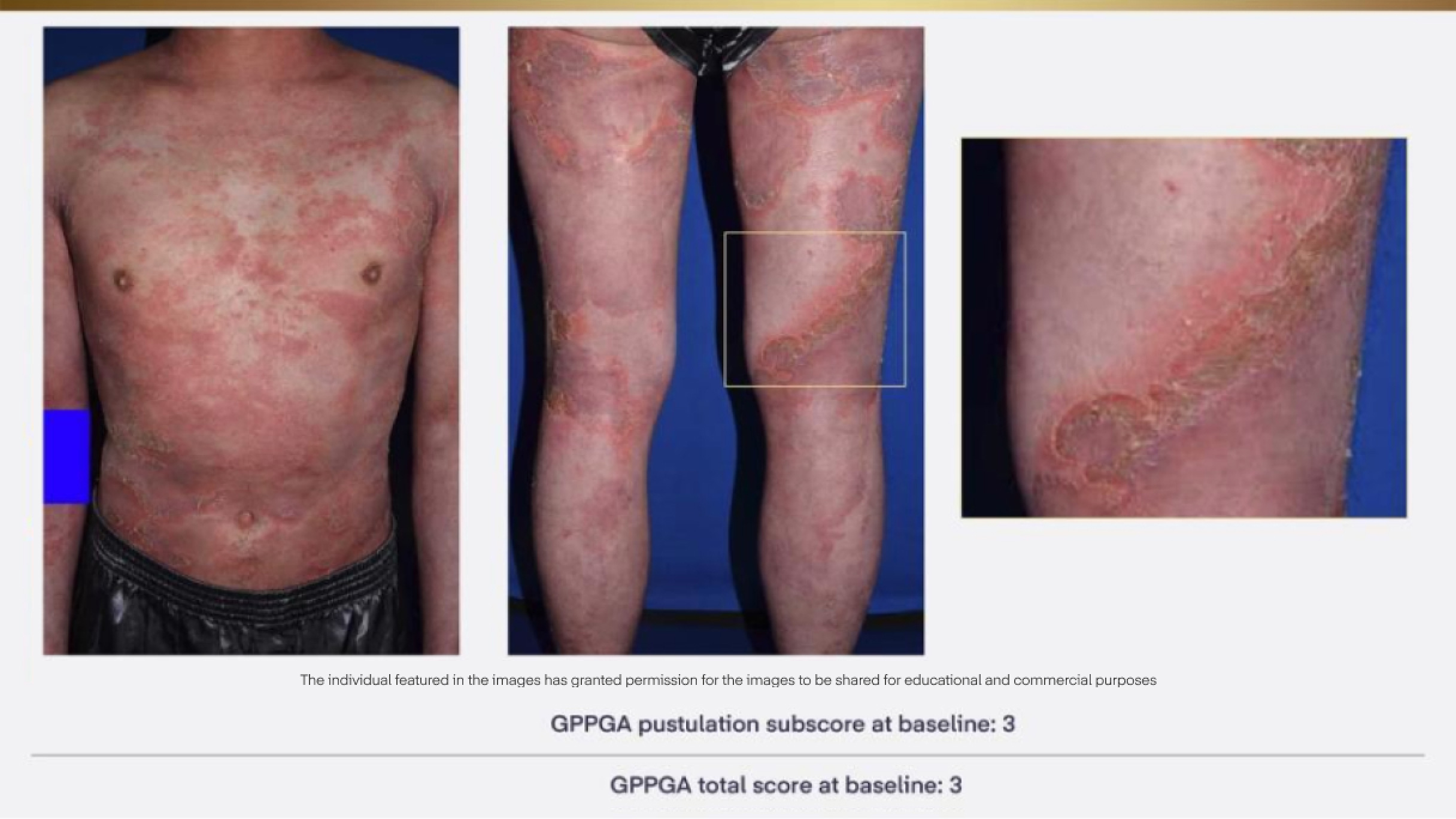
GPP=Generalised Pustular Psoriasis; GPPGA=Generalised Pustular Psoriasis Physician’s Global Assessment.
GPP is not plaque psoriasis3,7,8,13,14
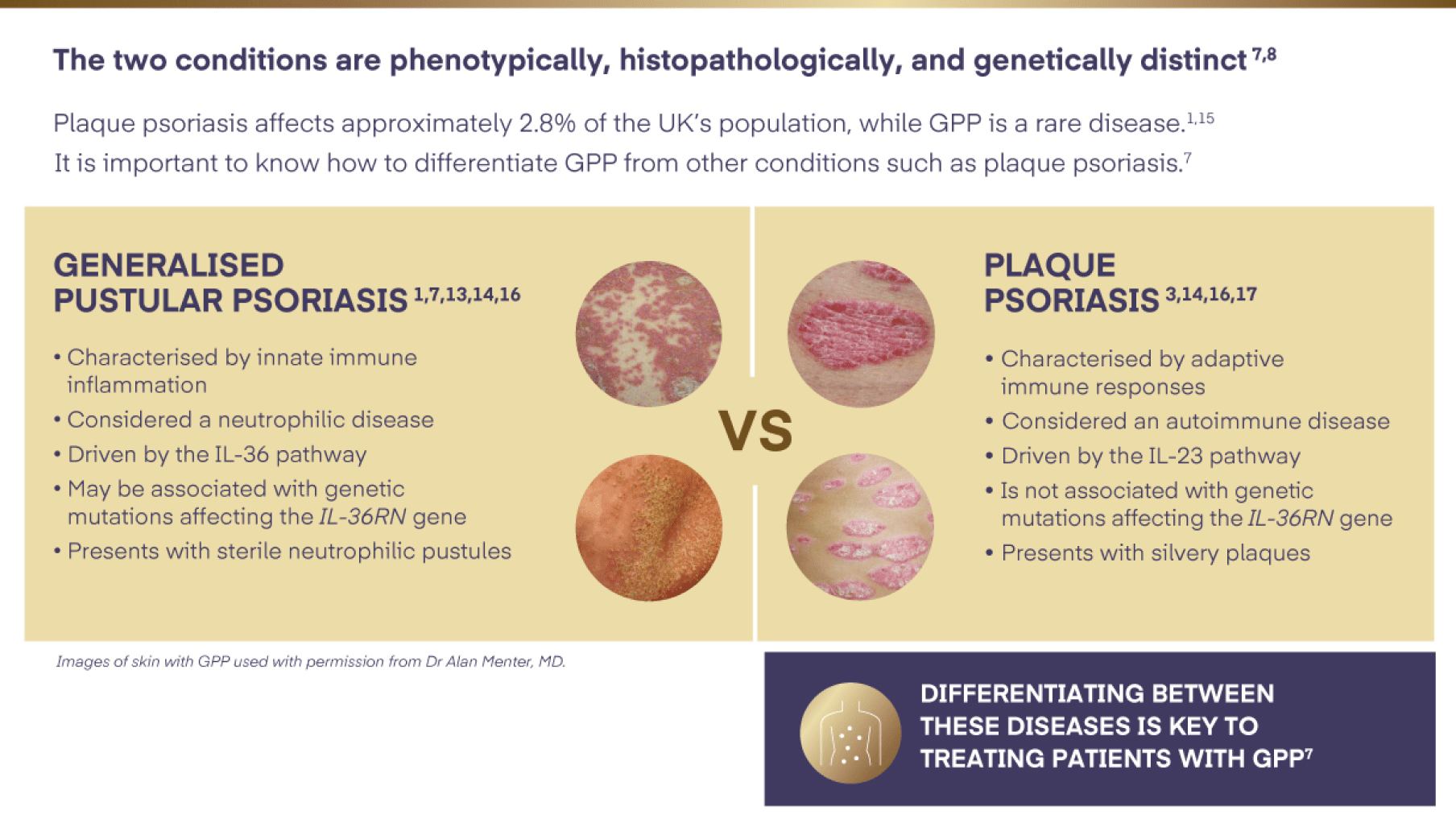
GPP=Generalised Pustular Psoriasis; IL-23=interleukin-23; IL-36=interleukin-36; IL-36RN =interleukin-36 receptor antagonist.
GPP has distinct characteristics6-8
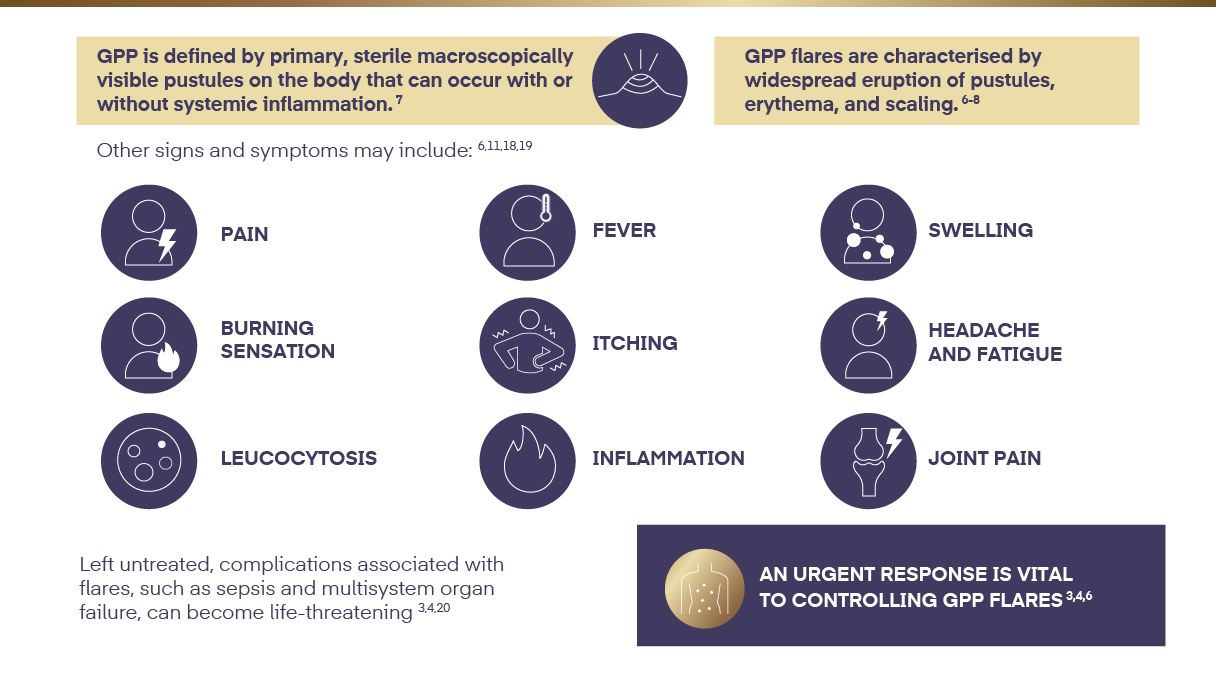
GPP=Generalised Pustular Psoriasis.
References
- Choon SE, Lebwohl MG, Marrakchi S, et al. Study protocol of the global Effisayil 1 phase II, multicentre, randomised, double-blind, placebo-controlled trial of spesolimab in patients with generalised pustular psoriasis presenting with an acute flare. BMJ Open. 2021;11(3):e043666.
- Kharawala S, Golembesky AK, Bohn RL, Esser D. The clinical, humanistic, and economic burden of generalised pustular psoriasis: a structured review. Expert Rev Clin Immunol. 2020;16(3):239-252.
- Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalised pustular psoriasis. Expert Rev Clin Immunol. 2019;15(9):907-919.
- Ly K, Beck KM, Smith MP, Thibodeaux Q, Bhutani T. Diagnosis and screening of patients with generalised pustular psoriasis. Psoriasis (Auckl). 2019;9:37-42.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178(3):614-618.
- Strober B, Kotowsky N, Medeiros R, et al. Unmet medical needs in the treatment and management of generalised pustular psoriasis flares: evidence from a survey of Corrona registry dermatologists. Dermatol Ther (Heidelb). 2021;11(2):529-541.
- Navarini AA, Burden AD, Capon F, et al; for the ERASPEN Network. European consensus statement on phenotypes of pustular psoriasis. J Eur Acad Dermatol Venereol. 2017;31(11):1792-1799.
- Benjegerdes KE, Hyde K, Kivelevitch D, Mansouri B. Pustular psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl). 2016;6:131-144.
- Sampogna F, Tabolli S, Söderfeldt B, Axtelius B, Aparo U, Abeni D; for the IMPROVE Investigators. Measuring quality of life of patients with different clinical types of psoriasis using the SF-36. Br J Dermatol. 2006;154(5):844-849.
- Kotowsky N, Gao R, Singer D, Garry E, Golembesky AK. Healthcare resource utilization (HCRU) in patients with generalised pustular psoriasis (GPP): a claims database study. Value in Health. 2020;23(suppl 1):S333-S334. Abstract: PRO29.
- Skalicky A, Rentz A, Esser D, Thoma C, Gloede T. Symptom experience of patients with generalised pustular psoriasis (GPP). Value in Health. 2020;23(suppl 1):S345. Abstract: PRO89.
- SPEVIGO® Data on File SPE-23-07.
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140(1):109-120.
- Liang Y, Sarkar MK, Tsoi LC, Gudjonsson JE. Psoriasis: a mixed autoimmune and autoinflammatory disease. Curr Opin Immunol. 2017;49:1-8.
- Springate DA, Parisi R, Kontopantelis E, et al. Incidence, prevalence and mortality of patients with psoriasis: a U.K. population-based cohort study. Br J Dermatol. 2017;176:650-658.
- Furue K, Yamamura K, Tsuji G, et al. Highlighting interleukin-36 signalling in plaque psoriasis and pustular psoriasis. Acta Derm Venereol. 2018;98(1):5-13.
- Pföhler C, Muller CSL, Vogt T. Psoriasis vulgaris and psoriasis pustulosa - epidemiology, quality of life, comorbidities and treatment. Curr Rheumatol Rev. 2013;9(1):2-7.
- Bachelez H. Pustular psoriasis: the dawn of a new era. Acta Derm Venereol. 2020;100(3):adv00034.
- Shah M, Al Aboud DM, Crane JS, Kumar S. Pustular psoriasis. StatPearls. Updated August 10, 2020. https://www.ncbi.nlm.nih.gov/books/NBK537002/ Accessed June 2024.
- Choon SE, Lai NM, Mohammad NA, Nanu NM, Tey KE, Chew SF. Clinical profile, morbidity, and outcome of adult-onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol. 2014;53(6):676-684.
PC-GB-109363 V2 | March 2025

