Spevigo (spesolimab) has a conditional marketing authorisation* for the treatment of generalised pustular psoriasis (GPP) flares in adults and adolescents from 12 years of age as monotherapy
▼This medicinal product is subject to additional monitoring. * Additional efficacy and safety data are being collected.
Click here for prescribing information.
†NICE approval as of 18 June 2025. Market availability as of November 2023.
Adults with generalised pustular psoriasis (GPP) flares can now be treated with SPEVIGO® (spesolimab), as NICE recommends1
SPEVIGO® is the first licensed treatment for GPP flares in the UK,2-5 and is now recommended by NICE for the treatment of GPP flares in adults.
NICE recommendation1
Spesolimab is recommended as an option for treating GPP flares in adults, only if it is used to treat:
Initial moderate to severe flares that have:
- A Generalised Pustular Psoriasis Physician Global Assessment (GPPGA) total score of 3 or more (at least moderate), and
- Fresh pustules (new appearance or worsening of existing pustules), and
- A GPPGA pustulation subscore of at least 2 (at least mild), and
- At least 5% of body surface area covered with erythema (abnormal redness of the skin or mucous membranes) and the presence of pustules.
Subsequent flares
Defined by a GPPGA pustulation subscore of 2 or more (at least mild), if the last flare was treated with spesolimab and resolved to a GPPGA pustulation subscore 0 or 1 (clear or almost clear skin).
Second dose for unresolved flares
A second dose of spesolimab can be used after 8 days only if a flare has not resolved to a GPPGA pustulation subscore of 0 or 1.
Take into account how skin colour could affect the GPPGA score and make any adjustments needed.
These recommendations are not intended to affect treatment with spesolimab that was started in the NHS before this guidance was published. People having treatment outside this recommendation may continue without change to the funding arrangements in place for them before this guidance was published, until they and their NHS healthcare professional consider it appropriate to stop.
How do we evaluate GPP patients?
Watch Prof. Richard Warren explain GPP assessment tools that can be used to measure disease severity and treatment outcomes.

Why the committee made these recommendations1
There is no licensed standard care for GPP flares. Usual treatment includes ciclosporin, acitretin and biological treatments used to treat other forms of psoriasis.
The licence for spesolimab does not include definitions for initial or subsequent GPP flare severity. For this evaluation, the severity definition used for initial flares is based on the criteria used in the clinical trial. The severity definition used for treating subsequent flares and retreating initial and subsequent flares are based on the criteria used in the economic model. Clinical advice is that these definitions align with how spesolimab would be used in clinical practice.
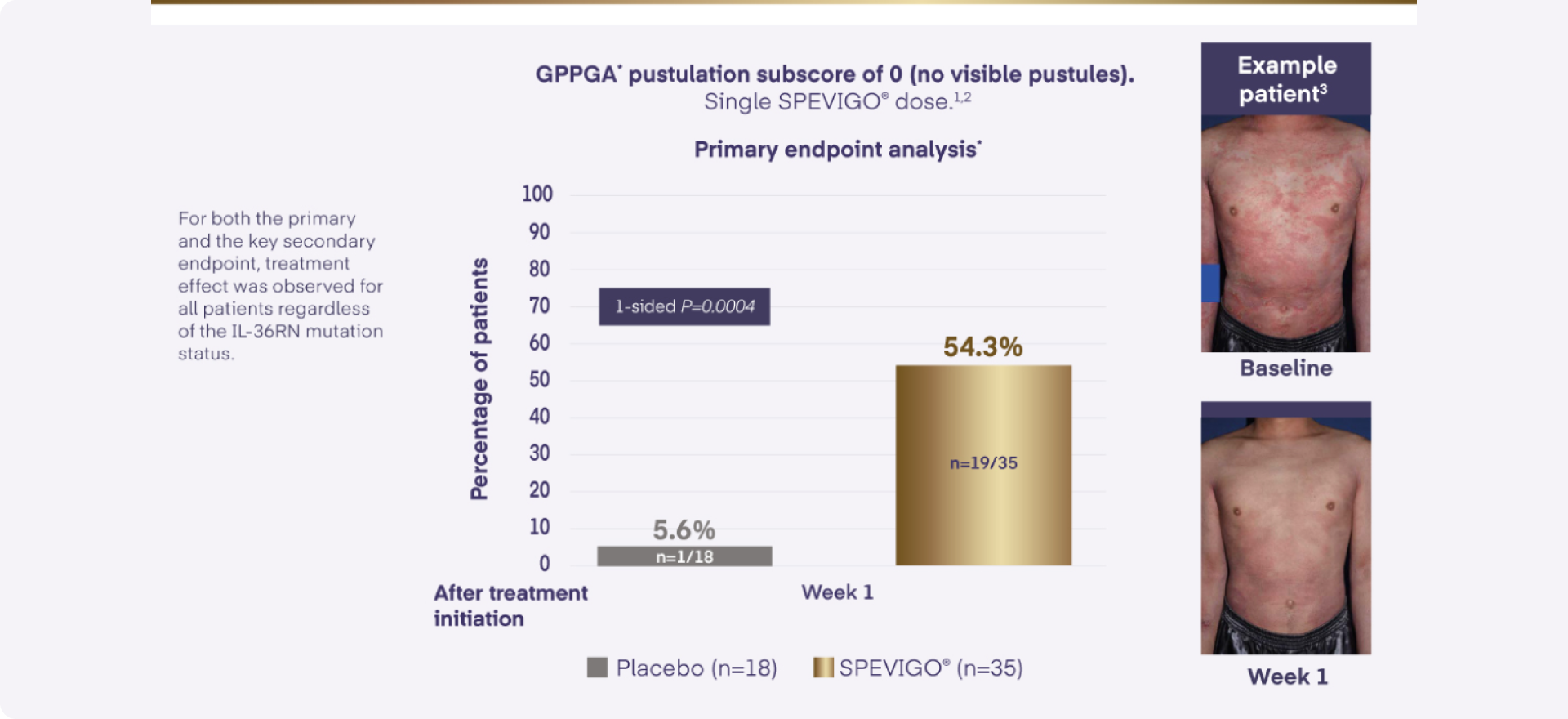
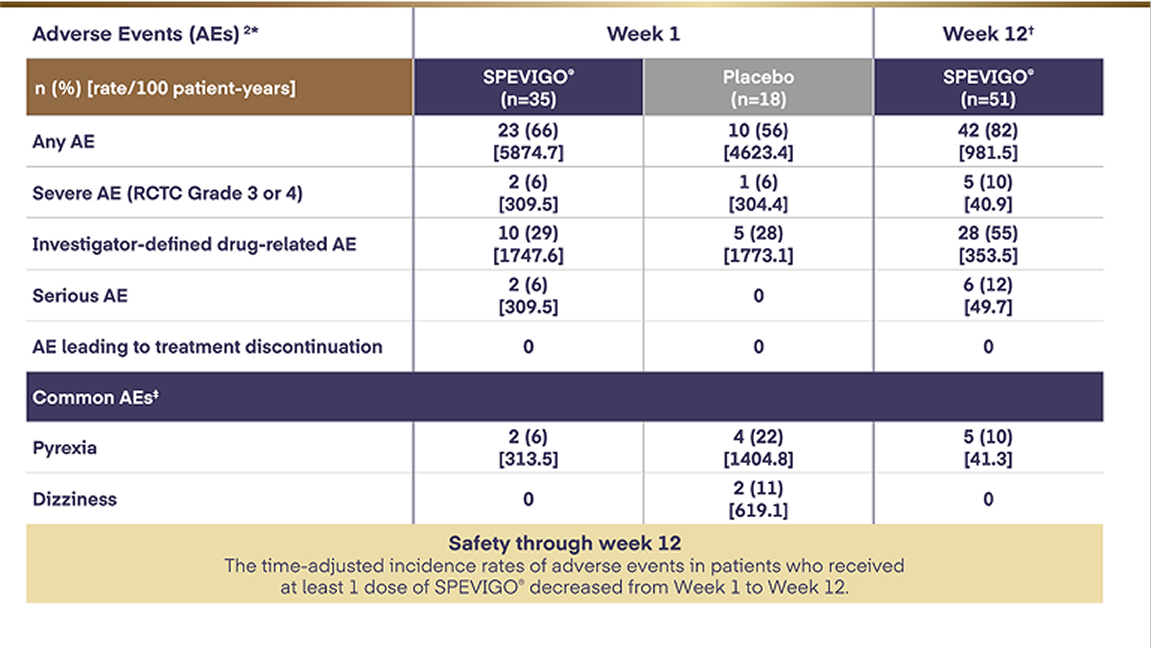
Clinical trial evidence shows that spesolimab resolves moderate to severe GPP flares faster than placebo. But it is uncertain how it affects the proportion of people who need hospital and intensive care admissions, or the length of hospital stays. There is no evidence comparing spesolimab with usual treatments used for GPP flares in the NHS.
The cost-effectiveness estimates for spesolimab are uncertain. This is because of uncertainties with the clinical evidence and assumptions used in the economic model around inpatient and intensive care admissions and spesolimab’s long-term effects. But there are also potential benefits not captured in the model. These include the potential for people to have fewer biological treatments for future GPP flares if they have spesolimab compared with usual treatment.
When taking this into consideration, the most likely cost-effectiveness estimates for spesolimab are within the range that NICE considers an acceptable use of NHS resources. So, it is recommended.
Clicking on this button will take you to a third party website not managed by Boehringer.
Learn more about how SPEVIGO® can be used to treat GPP flares
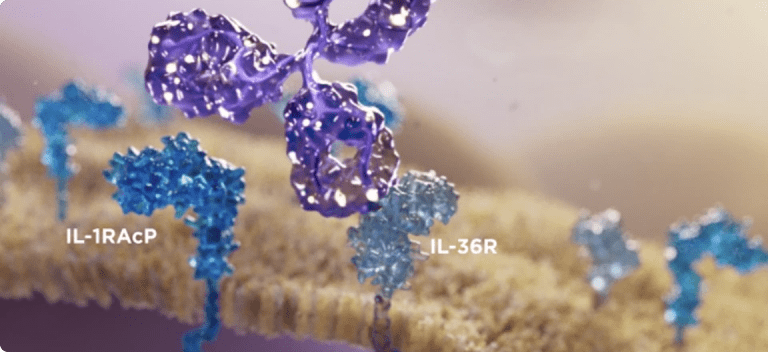
The only approved treatment to block the key inflammatory pathway in GPP by targeting IL-36 receptor1,5,6
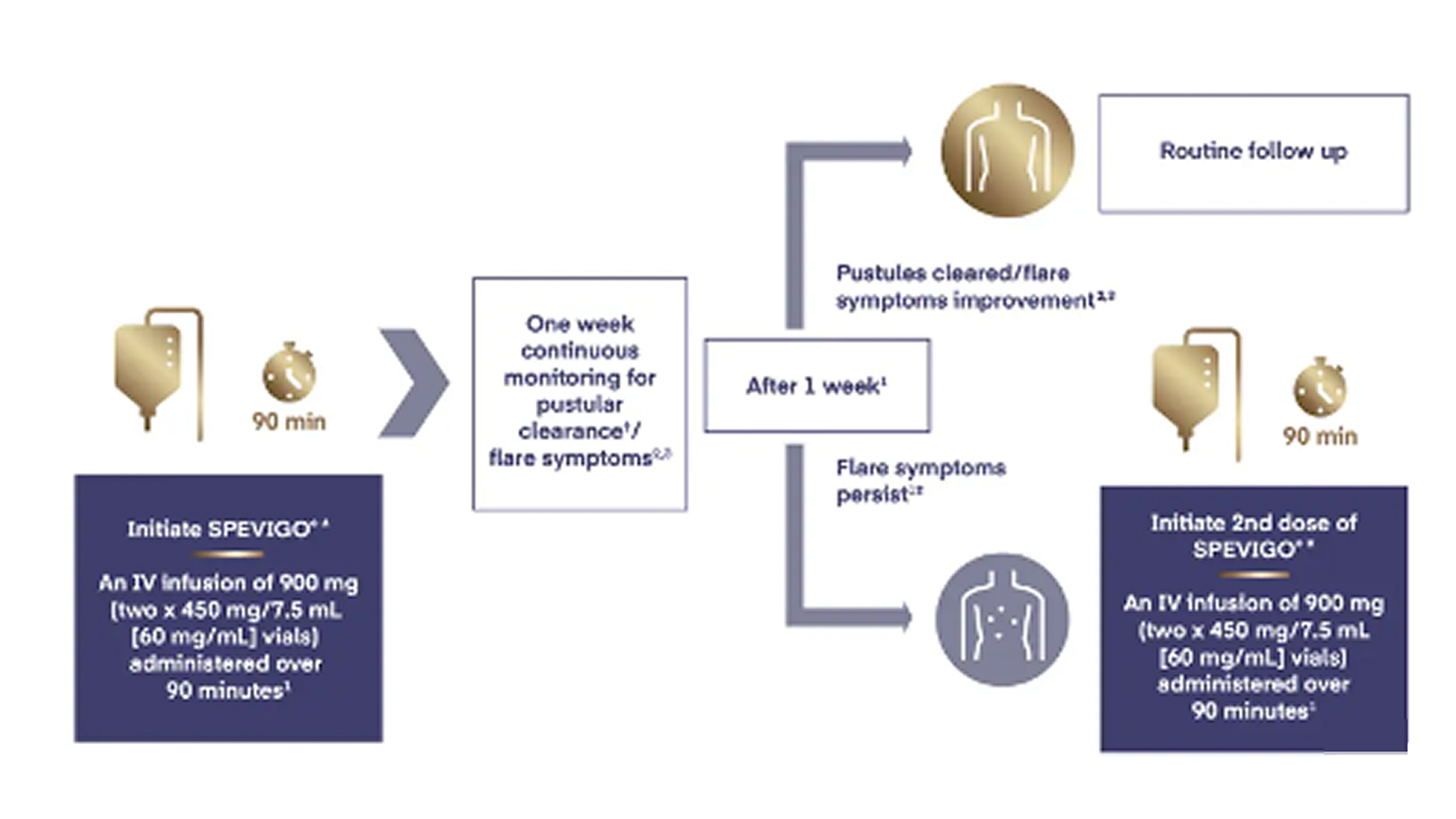
Treat GPP flares with SPEVIGO®1,5
Learn more about SPEVIGO® and how to administer it
Abbreviations:
GPP: Generalised pustular psoriasis; GPPGA: Generalised Pustular Psoriasis Physician Global Assessment; NICE: National Institute of Health and Care Excellence
References
- National Institute for Health and Care Excellence (NICE). Technology appraisal guidance TA1070. Spesolimab for treating generalised pustular psoriasis flares, 18 June 2025. Available at https://www.nice.org.uk/guidance/TA1070/. Accessed June 2025.
- SPEVIGO® Summary of Product Characteristics. Boehringer Ingelheim.
- Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalized pustular psoriasis. Expert Rev Clin Immunol. 2019;15(9):907-919.
- Ly K, Beck KM, Smith MP, Thibodeaux Q, Bhutani T. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37-42.
- Bachelez H, Choon SE, Marrakchi S, et al; for the Effisayil 1 trial investigators. Trial of spesolimab for generalised pustular psoriasis. N Engl J Med. 2021;385(26):2431-2440.
- Hawkes JE, Visvanathan S, Krueger JG, et al. The role of the interleukin-36 axis in generalized pustular psoriasis: a review of the mechanism of action of spesolimab. Front Immunol. 2023;14:1292941.
PC-GB-111613 V1 | June 2025