Spevigo (spesolimab) has a conditional marketing authorisation for the treatment of generalised pustular psoriasis (GPP) flares in adults and adolescents from 12 years of age as monotherapy
▼This medicinal product is subject to additional monitoring. * Additional efficacy and safety data are being collected.
Click here for prescribing information.
SPEVIGO®▼ (spesolimab) achieved the primary endpoint within the EffisayilTM 1 study1,2
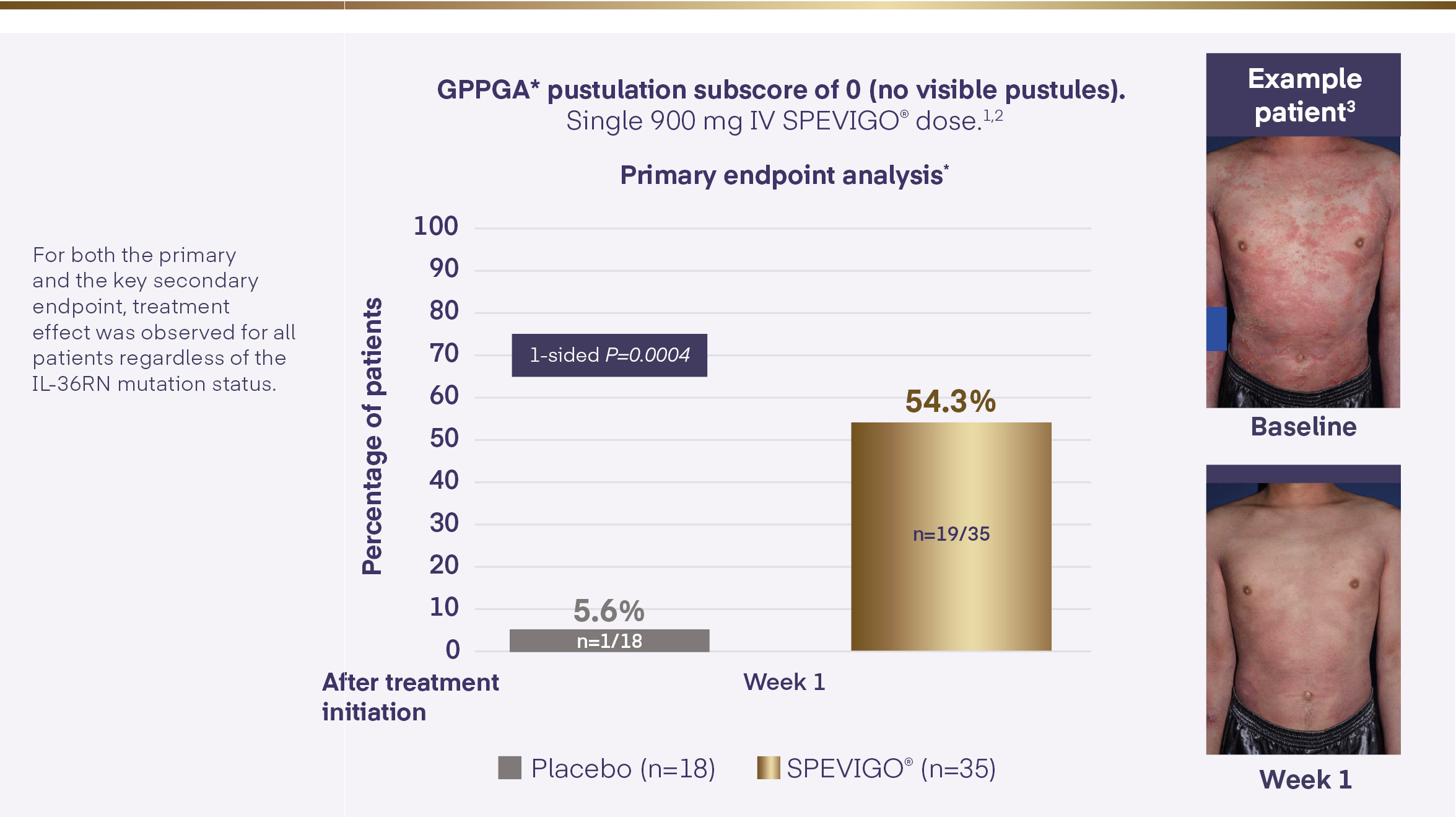
*Missing values or any use of other medication for GPP within the first week of the trial was regarded as non-response for the analysis of the endpoint.2
The individual featured in the images has granted permission for the images to be shared for educational and commercial purposes
GPP=Generalised Pustular Psoriasis; GPPGA=Generalised Pustular Psoriasis Physician Global Assessment; IL-36RN=interleukin-36 receptor antagonist; IV=intravenous.
SPEVIGO® achieved the secondary endpoint within the EffisayilTM 1 study1,2
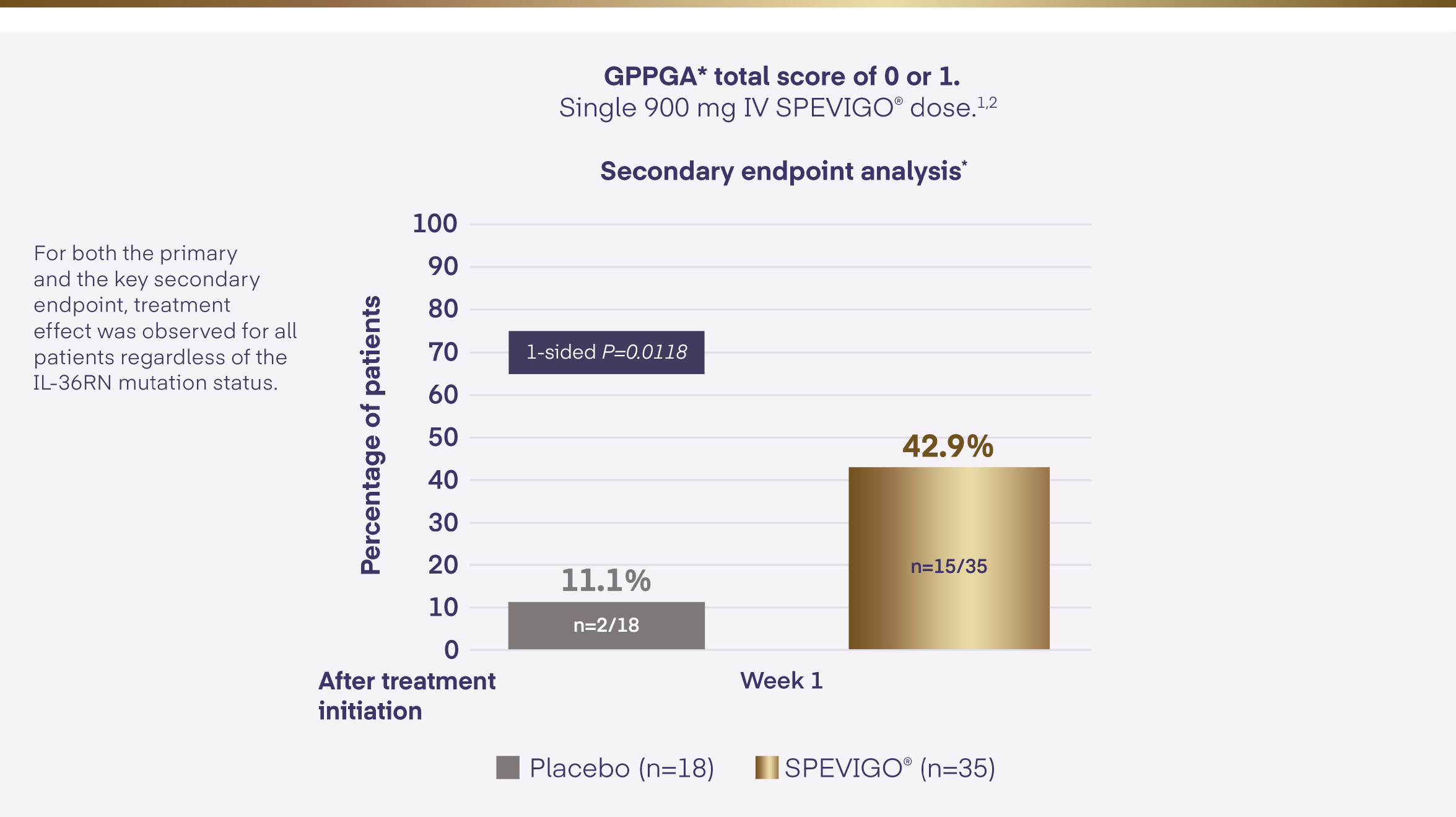
*Missing values or any use of other medication for GPP within the first week of the trial was regarded as non-response for the analysis of the endpoint.2
GPP=Generalised Pustular Psoriasis; GPPGA=Generalised Pustular Psoriasis Physician Global Assessment; IL-36RN=interleukin-36 receptor antagonist; IV=intravenous.
In EffisayilTM 1, a GPPGA score of 0 or 1 (clear or almost clear skin) was sustained with SPEVIGO® for the 12 weeks study period2
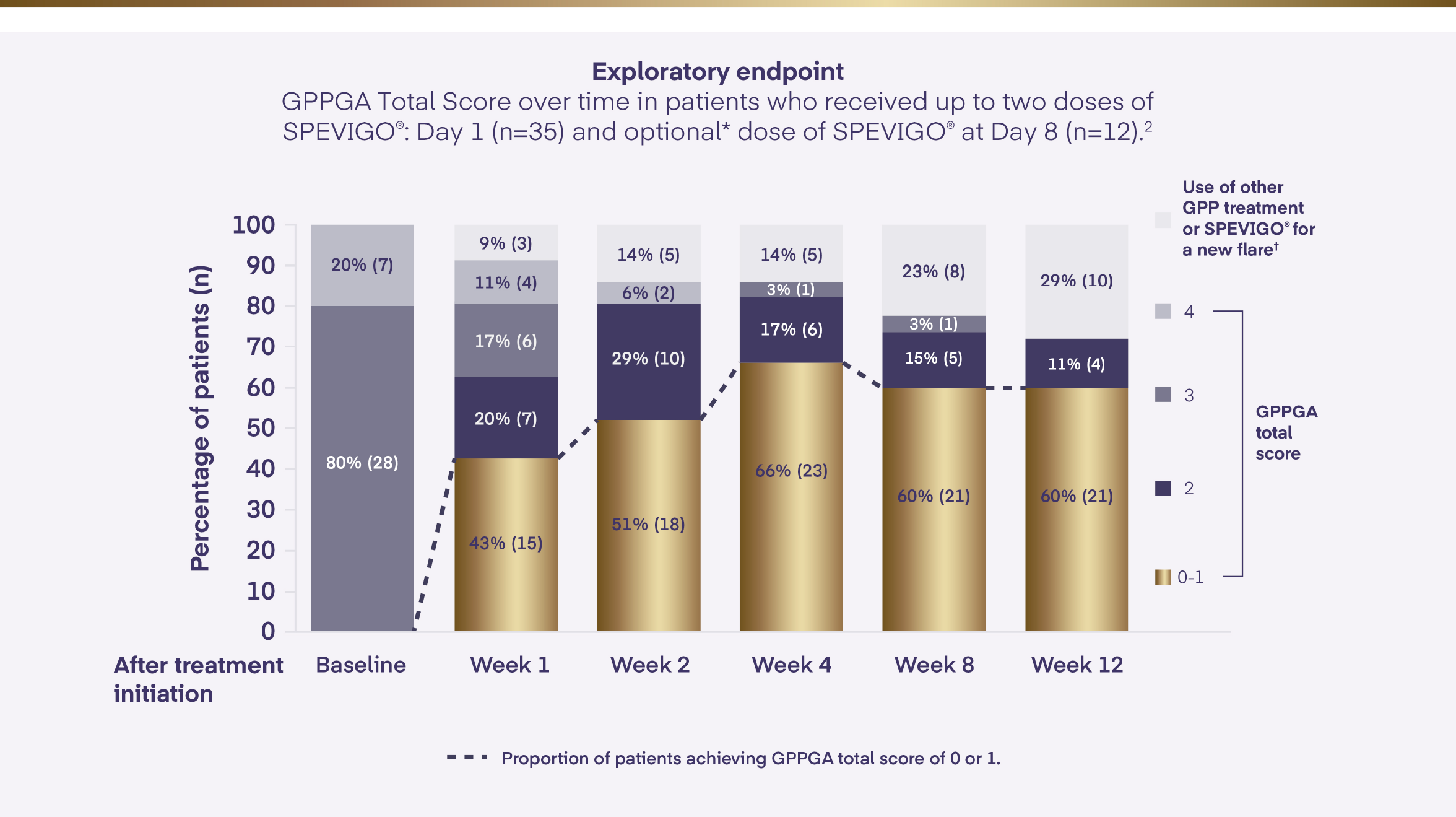
*At day 8, patients with GPPGA total score ≥2 and GPPGA pustulation subscore ≥2 could receive open-label SPEVIGO®.
†Defined as an increase of ≥2 points in both the GPPGA total score and the pustulation subscore after a GPPGA total score of 0 or 1 had been reached.
GPP=Generalised Pustular Psoriasis; GPPGA=Generalised Pustular Psoriasis Physician Global Assessment.
References
- SPEVIGO® Summary of Product Characteristics. Boehringer Ingelheim.
- Bachelez H, Choon SE, Marrakchi S, et al; for the Effisayil 1 trial investigators. Trial of spesolimab for generalised pustular psoriasis. N Engl J Med. 2021;385(26):2431-2440.
- SPEVIGO® Data on File SPE-23-07.
PC-GB-109366 V2 | March 2025

