Spevigo (spesolimab) has a conditional marketing authorisation for the treatment of generalised pustular psoriasis (GPP) flares in adults and adolescents from 12 years of age as monotherapy
▼This medicinal product is subject to additional monitoring. * Additional efficacy and safety data are being collected.
Click here for prescribing information.
SPEVIGO®▼ (spesolimab) Product Overview1
SPEVIGO® has a conditional marketing authorisation* for the treatment of GPP flares in adults and adolescents from 12 years of age as monotherapy.
SPEVIGO® is supplied as a sterile concentrate solution in a single-dose vial.
Each vial contains 450 mg of SPEVIGO® in 7.5-mL (60-mg/mL) concentrate solution for infusion.
In adults and adolescents from 12 years of age and weighing at least 40 kg, SPEVIGO® is administered by intravenous infusion as a single (two x 450 mg/7.5 mL vials) 900 mg dose.
If flare symptoms persist,† an additional 900 mg dose may be administered 1 week after the initial dose.
In adolescents from 12 years of age weighing ≥30 and <40 kg, SPEVIGO® is administered by intravenous infusion as a single (one x 450 mg/7.5 mL vial) 450 mg dose.
If flare symptoms persist,† an additional 450 mg dose may be administered 1 week after the initial dose.
Clinical data for treatment of subsequent flares is very limited.
*Additional efficacy and safety data are being collected.
†Persistent flare defined as ≥2-point GPPGA total score and ≥2-point GPPGA pustulation subscore.2
GPPGA=Generalised Pustular Psoriasis Physician's Global Assessment.
Adults and adolescents from 12 years of age and weighing at least 40 kg
The recommended dose of SPEVIGO® in adults and adolescents from 12 years of age and weighing at least 40 kg is a single continuous IV infusion of 900 mg (two vials of 450 mg) administered over 90 minutes.1
If flare symptoms persist, a single additional IV infused dose of 900 mg (two vials of 450 mg) may be administered 1 week after the initial dose.1
Treatment should be initiated and supervised by physicians experienced in the management of patients with inflammatory skin conditions.1
Clinical data for treatment of subsequent flares is very limited.1
Clinical data for concomitant use of other GPP treatments with SPEVIGO® is limited.1
SPEVIGO® should not be used in combination with other GPP treatments.1
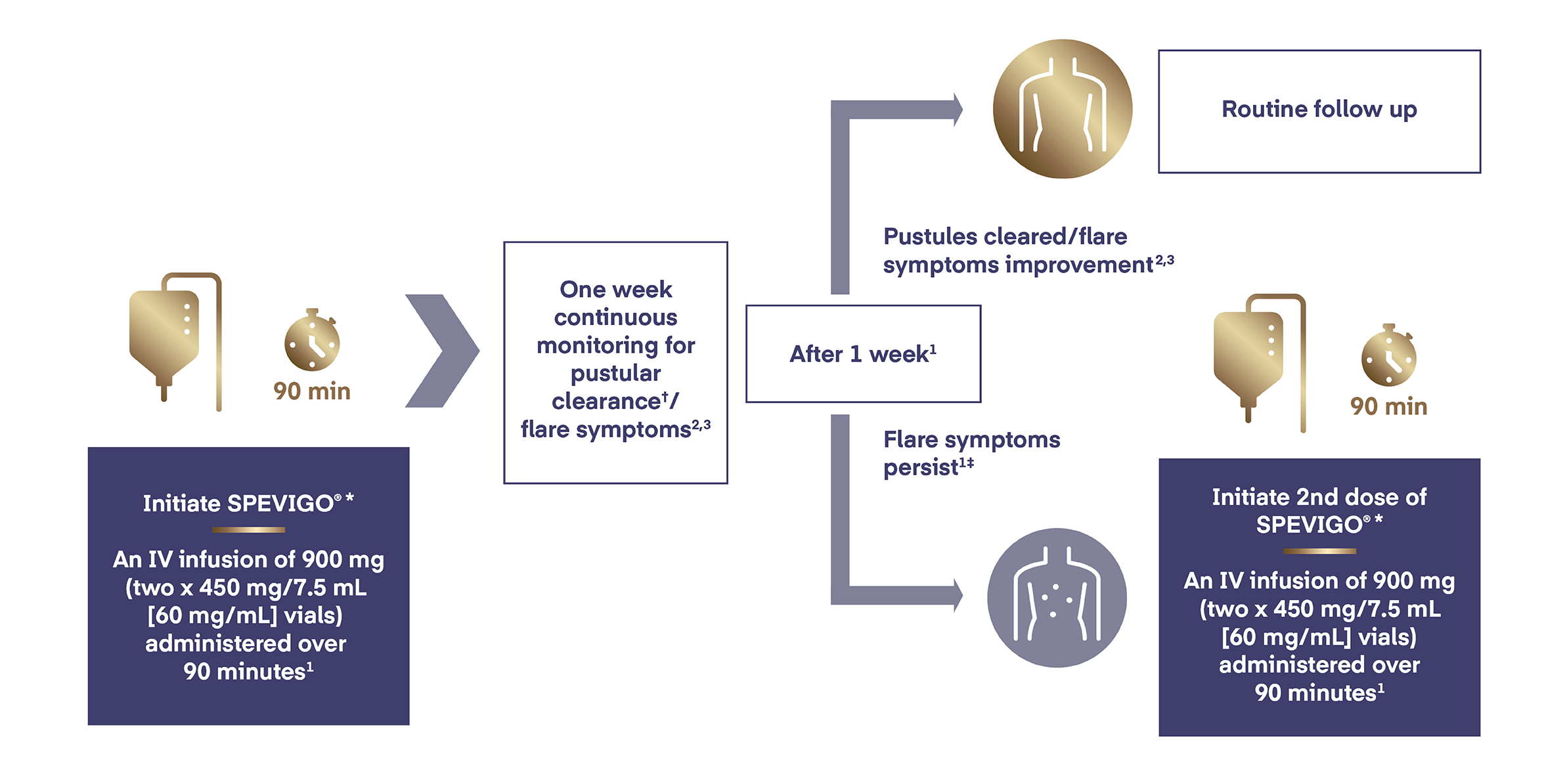
*SPEVIGO® should not be administered as an intravenous push or bolus. Following dilution with sodium chloride 9 mg/mL (0.9%) solution for injection, SPEVIGO® is administered as a continuous intravenous infusion through an intravenous line containing a sterile, non-pyrogenic, low protein binding in-line filter (pore size of 0.2 micron) over 90 minutes. No other infusion should be administered in parallel via the same intravenous access. In the event that the infusion is slowed or temporarily stopped, the total infusion time (including stop time) should not exceed 180 minutes.1
†Pustular clearance defined as GPPGA pustulation subscore of 0 (no visible pustules).2
‡Flare persistence defined as GPPGA total score ≥2 and GPPGA pustulation subscore ≥2.2
GPP=Generalised Pustular Psoriasis; GPPGA=Generalised Pustular Psoriasis Physician Global Assessment; IV=intravenous; SmPC=Summary of Product Characteristics.
Adolescents from 12 years of age weighing ≥30 and <40 kg
SPEVIGO® has not been studied in patients weighing less than 40 kg. Based on pharmacokinetic modelling and simulation, the recommended dose of SPEVIGO® in adolescents from 12 years of age and weighing ≥30 and <40 kg is a single continuous IV infusion of 450 mg (one vial of 450 mg) administered over 90 minutes.1
If flare symptoms persist, a single additional IV infused dose of 450 mg (one vial of 450 mg) may be administered 1 week after the initial dose.1
Treatment should be initiated and supervised by physicians experienced in the management of patients with inflammatory skin diseases.1
Clinical data for treatment of subsequent flares is very limited.1
Clinical data for concomitant use of other GPP treatments with SPEVIGO® is limited.1
SPEVIGO® should not be used in combination with other GPP treatments.1
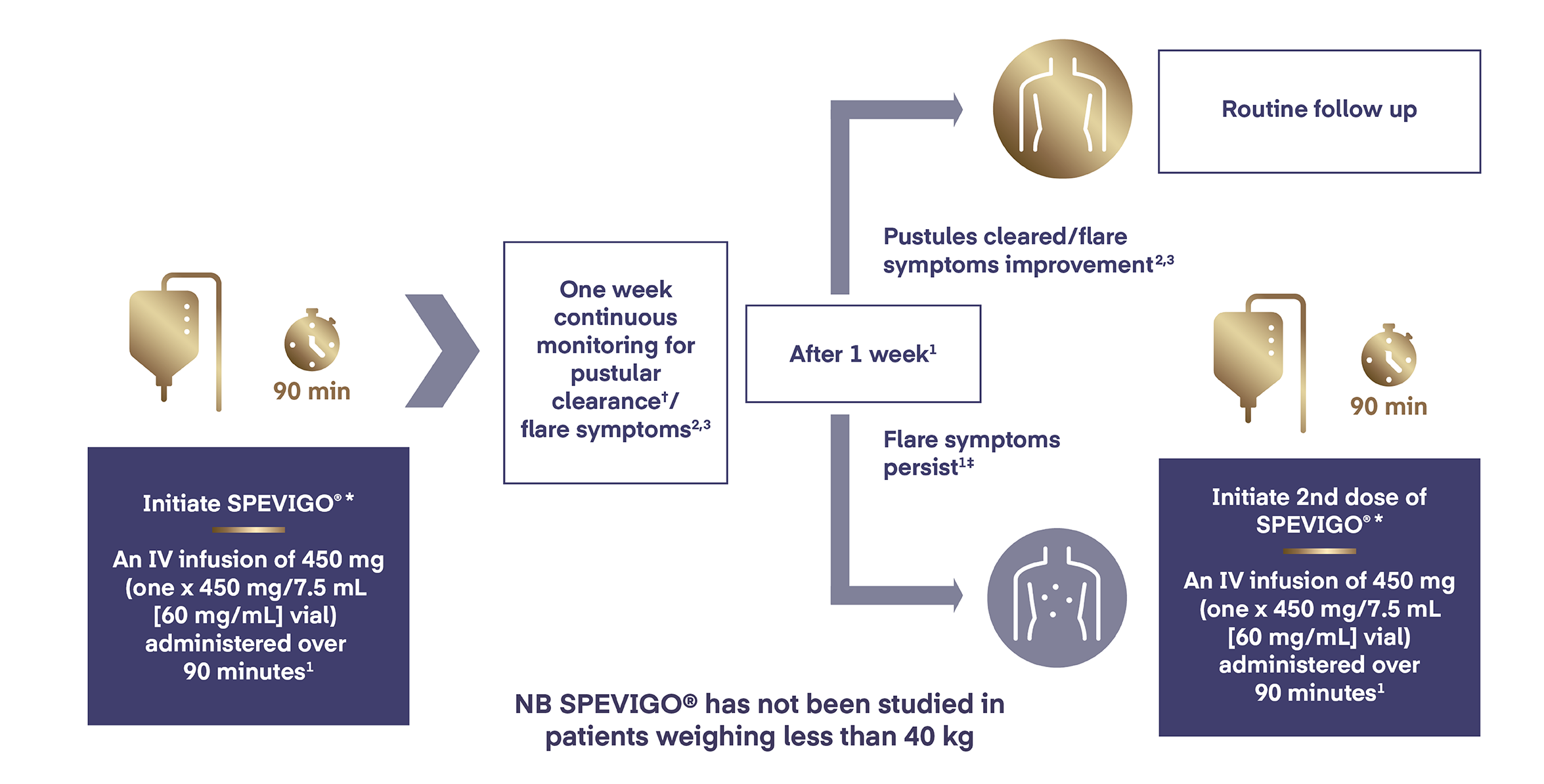
*SPEVIGO® should not be administered as an intravenous push or bolus. Following dilution with sodium chloride 9 mg/mL (0.9%) solution for injection, SPEVIGO® is administered as a continuous intravenous infusion through an intravenous line containing a sterile, non‑pyrogenic, low protein binding in-line filter (pore size of 0.2 micron) over 90 minutes. No other infusion should be administered in parallel via the same intravenous access. In the event that the infusion is slowed or temporarily stopped, the total infusion time (including stop time) should not exceed 180 minutes.1
†Pustular clearance defined as GPPGA pustulation subscore of 0 (no visible pustules).2
‡Flare persistence defined as GPPGA total score ≥2 and GPPGA pustulation subscore ≥2.2
GPP=Generalised Pustular Psoriasis; GPPGA=Generalised Pustular Psoriasis Physician Global Assessment; IV=intravenous; SmPC=Summary of Product Characteristics.
Storage of SPEVIGO®1
Storage of SPEVIGO® sterile concentrate
Long-term storage
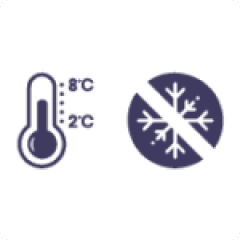
Unopened vials should be refrigerated (2°C to 8°C). The shelf life of an unopened vial is three years unless the vial has a shorter expiry date. Do not freeze.

Store in the original package in order to protect from light.
Short-term storage
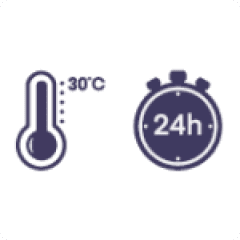
Prior to use, the unopened vial may be kept at temperatures up to 30°C for up to 24 hours.

Store in the original package in order to protect from light.
Storage of diluted solution
If not administered immediately, store the diluted solution under refrigeration at 2°C to 8°C for up to 24 hours.

For the time between preparation and start of administration, protect solution from light.
Preparation of SPEVIGO® for IV Infusion1
SPEVIGO® is for single-use only.
The vial should be visually inspected before use. SPEVIGO® is a colourless to slightly brownish-yellow, clear to slightly opalescent solution. If the solution is cloudy, discoloured, or contains large or coloured particulates, the vial should be discarded.

Aseptic technique must be used to prepare the solution for infusion.

For the recommended dose of 900 mg, draw and discard 15 mL from a 100 mL container of sterile 0.9% sodium chloride solution and replace slowly with 15 mL SPEVIGO® sterile concentrate (complete content from two vials of 450 mg/7.5 mL).

For the recommended dose of 450 mg, draw and discard 7.5 mL from a 100 mL container of sodium chloride 9 mg/mL (0.9%) solution for injection and replace slowly with 7.5 mL SPEVIGO® sterile concentrate (one vial of 450 mg/7.5 mL).
Mix gently before use. The diluted SPEVIGO® infusion solution should be used immediately.
IV=intravenous.
Administration of SPEVIGO®1

SPEVIGO® is for intravenous infusion only. It should not be administered as an intravenous push or bolus.

SPEVIGO® must not be mixed with other medicinal products.

Following dilution with 0.9% sodium chloride solution for injection, SPEVIGO® is administered as a continuous intravenous infusion through an intravenous line containing a sterile, non-pyrogenic, low-protein binding in-line filter (pore size of 0.2 micron) over 90 minutes.

If the infusion is slowed or temporarily stopped, the total infusion time (including stop time) should not exceed 180 minutes.

A pre-existing intravenous line may be used for administration of diluted SPEVIGO®. The line must be flushed with sterile 0.9% sodium chloride solution prior to and at the end of infusion. No other infusion should be administered in parallel via the same intravenous access.

No incompatibilities have been observed between SPEVIGO® and infusion sets composed of PVC, PE, PP, polybutadiene and PUR, and in-line filter membranes composed of PES (neutral and positively charged) and positively charged PA.
PA=polyamide; PE=polyethylene; PES=polyethersulfone; PP=polypropylene; PUR=polyurethane; PVC=polyvinylchloride.
Hypersensitivity and infusion-related reactions
Hypersensitivity and infusion-related reactions may occur with monoclonal antibodies such as SPEVIGO®. Hypersensitivity may include immediate reactions, such as anaphylaxis, and delayed reactions, such as drug reaction with eosinophilia and systemic symptoms (DRESS).
Immediate hypersensitivity reactions, including anaphylactic reactions have been reported in patients treated with spesolimab.
If a patient develops signs of anaphylaxis or other serious hypersensitivity, SPEVIGO® treatment should be discontinued immediately, and appropriate treatment initiated.
If a patient develops mild or moderate infusion-related reactions, SPEVIGO® should be stopped and appropriate medical therapy considered (e.g., systemic antihistamines and/or corticosteroids).
Upon resolution of the reaction, the infusion may be restarted at a slower infusion rate with gradual increases to complete the infusion.
Post-infusion Patient Monitoring1

If flare symptoms persist, an additional 900 mg dose may be administered 1 week after the initial dose. Clinical data for treatment of subsequent flares is very limited.
Important post-infusion considerations
After SPEVIGO® treatment, patients should be monitored for signs and symptoms of active tuberculosis (TB).
Patients should be instructed to seek medical advice if signs or symptoms of clinically important infection occur after treatment with SPEVIGO®.
Hypersensitivity may include immediate reactions such as anaphylaxis and delayed reactions such as drug reaction with eosinophilia and systemic symptoms (DRESS).
Live vaccines should not be administered for at least 16 weeks after treatment with SPEVIGO®.
Encourage your patients to report any side effects
May increase the risk of infections.*
Hypersensitivity‡ and infusion-related reactions† may occur.
Other common side effects include pruritus and fatigue.
*The most commonly reported infections were urinary tract infection (common) and upper respiratory tract infection (common).
†Not reported in Effisayil™ 1.
‡Derived from open-label extension trials and post-marketing experience.
References
- SPEVIGO® Summary of Product Characteristics. Boehringer Ingelheim.
- Bachelez H, Choon SE, Marrakchi S, et al; for the Effisayil 1 Trial Investigators. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385(26):2431-2440.
- Choon SE, Lebwohl MG, Marrakchi S, et al. Study protocol of the global Effisayil 1 phase II, multicentre, randomized, double-blind, placebo-controlled trial of spesolimab in patients with generalized pustular psoriasis presenting with an acute flare. BMJ Open. 2021;11(3):e043666.
PC-GB-109368 V3 | July 2025

