Clinical Trial Data
Metalyse 25 mg is available for the thrombolytic treatment of AIS.1 It offers non-inferior efficacy and a comparable safety profile to Actilyse® (alteplase), but with simpler dosing, a faster, more convenient administration method, and a lower treatment cost per patient.*1–6 It is recommended, along with Actilyse, by the National Clinical Guideline for Stroke for the UK and Ireland as a treatment option for AIS within 4.5 hours of onset.7
* Based on the average weight of a UK stroke patient
Key clinical trials
Explore below to learn the key efficacy and safety outcomes for Metalyse 25 mg compared with Actilyse from the AcT, ATTEST-2 and EXTEND-IA TNK clinical trials.3–5
AcT: Metalyse 25 mg demonstrated comparable safety and non-inferior efficacy vs Actilyse.3
The phase III AcT trial compared the efficacy and safety of Metalyse 25 mg with Actilyse in adult patients with AIS presenting within 4.5 hours of onset.3
Patients randomly assigned to Metalyse 25 mg received a one-time decile-weight-tiered bolus dose of 0.25 mg/kg to a maximum of 25 mg. Those assigned to Actilyse received a total dose of 0.9 mg/kg to a maximum of 90 mg.3
The Metalyse 25 mg dose of 0.25 mg/kg was identified through phase II trials as the most appropriate when considering efficacy and safety data. The licensed posology, as used in the AcT trial, used decile-weight-tiered dosing seen in the table below.5,8–11
Weight-based dosing for Metalyse 25 mg used in the AcT trial.
| Patient's body weight (kg) | Tenecteplase (U) | Tenecteplase (mg) | Corresponding volume of reconstituted solution (ml) |
|---|---|---|---|
| <60 | 3,000 | 15.0 | 3.0 |
| ≥60 to <70 | 3,500 | 17.5 | 3.5 |
| ≥70 to <80 | 4,000 | 20.0 | 4.0 |
| ≥80 to <90 | 4,500 | 22.5 | 4.5 |
| ≥90 | 5,000 | 25.0 | 5.0 |
The benefits and risks of Metalyse 25 mg treatment should be carefully evaluated in patients who weigh 50 kg or less, due to limited availability of data in this group of patients.
Primary outcome: non-inferiority to Actilyse in excellent functional outcome3
Excellent functional outcome (defined as an mRS score of 0–1 at 90–120 days) was achieved by 36.9% (n=296/802) of patients who received Metalyse 25 mg vs 34.8% (n=266/765) of those who received Actilyse (unadjusted risk difference 2.1% [95% CI −2.6 to 6.9; non-inferiority margin: −5%]).3
The direction of the effect favoured Metalyse 25 mg, however, this was not statistically significant (unadjusted RR: 1.0 [95% CI 1.0 to 1.1]; P = 0.19).3
Results were consistent with no significant differences observed across all pre-specified subgroups, including age (<80 vs ≥80 years), sex, baseline stroke severity, symptom onset-to-needle time, large vessel occlusion, type of enrolling centre, and source registry.3 All subgroup analyses were exploratory.3
Distribution of the mRS scores at 90–120 days3
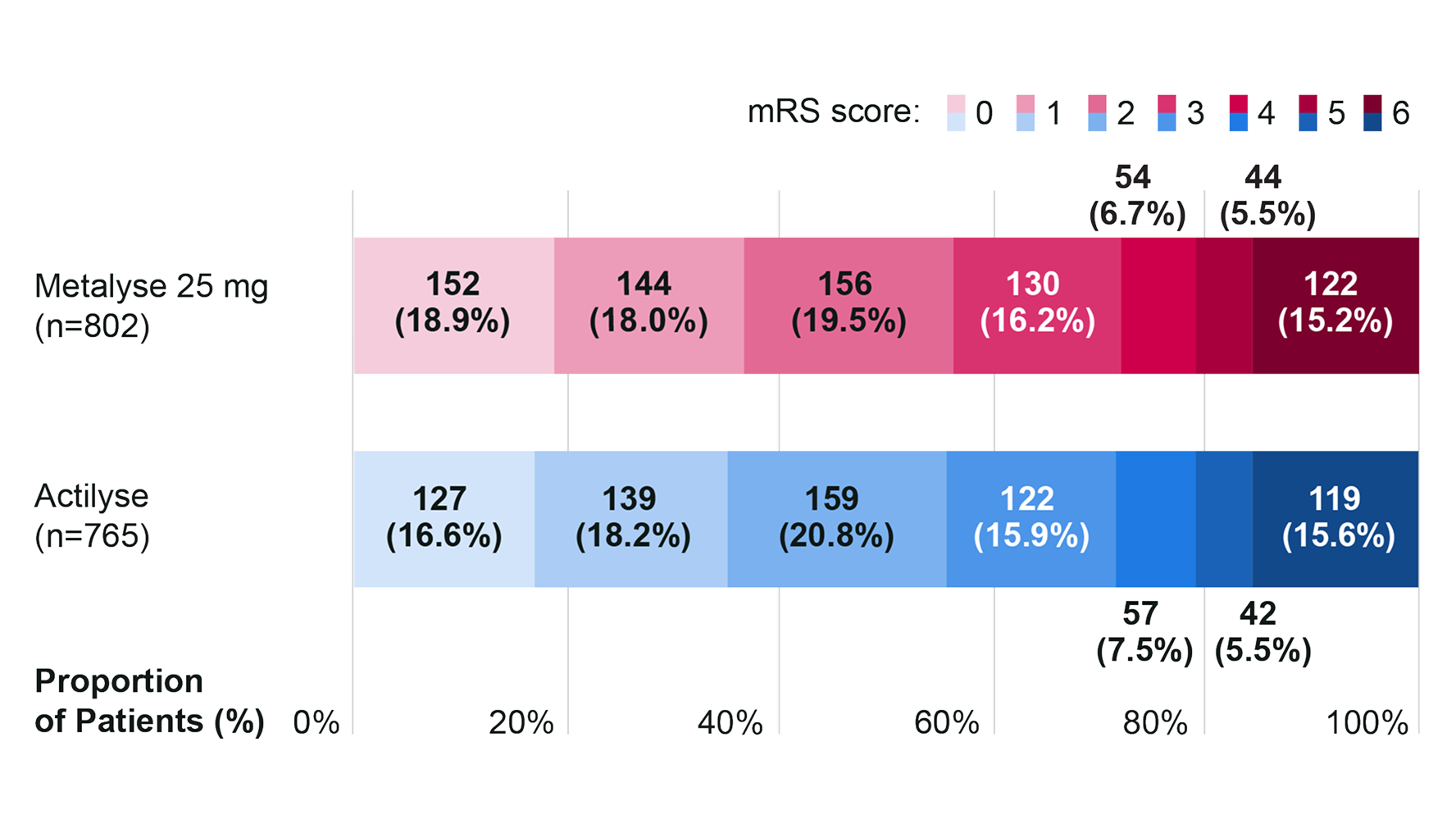
Adapted from Menon BK, et al. 2022.3
mRS scores range from 0 to 6, with 0 indicating no symptoms, 1 no clinically significant disability, 2 slight disability, 3 moderate disability, 4 moderately severe disability, 5 severe disability, and 6 death.
Metalyse 25 mg and Actilyse had comparable secondary outcomes3
mRS score of 0–2 at 90–120 days (n=1,567)
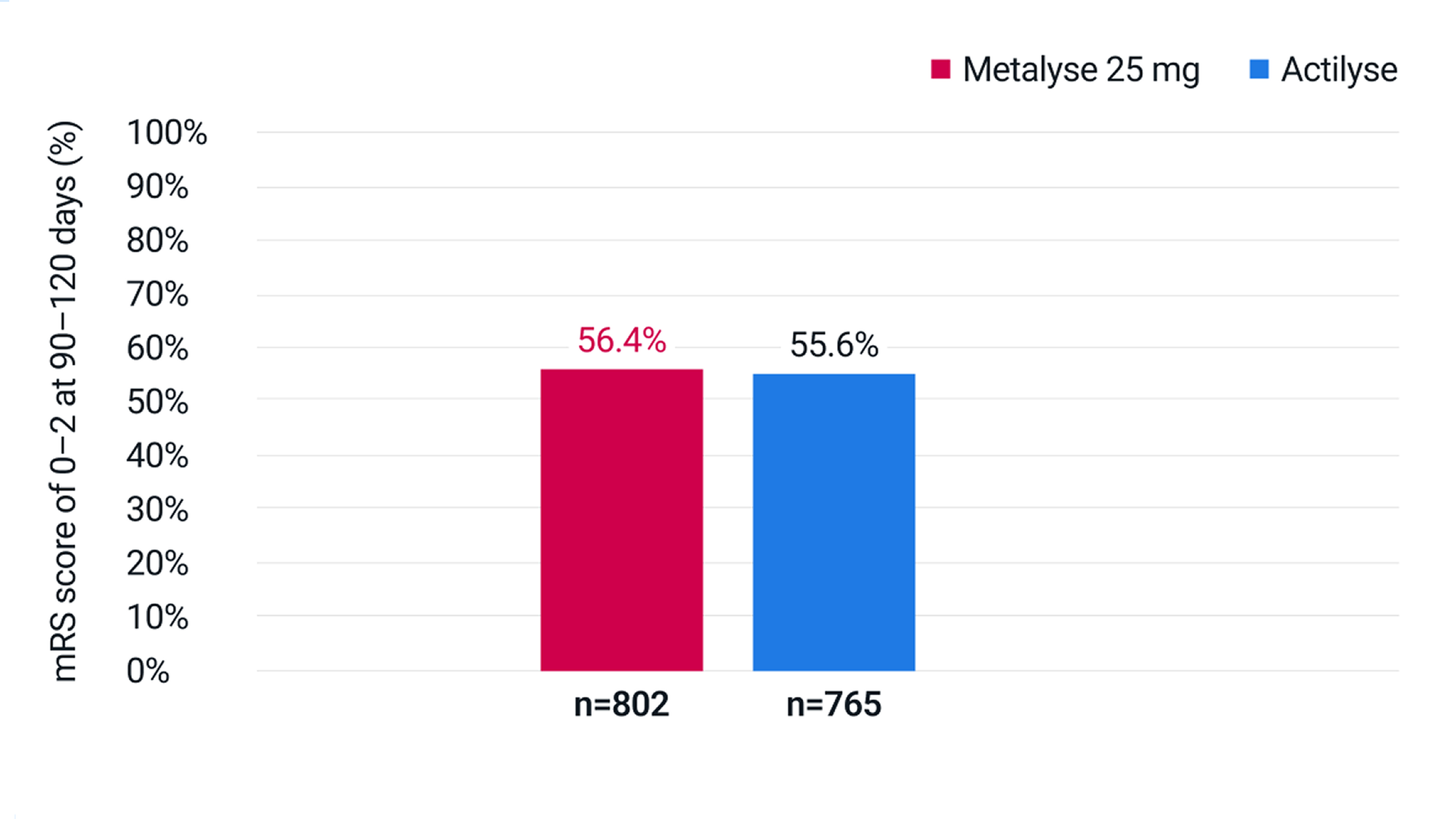
Adapted from Menon BK, et al. 2022.3
56.4% of patients receiving Metalyse 25 mg vs 55.6% of patients receiving Actilyse had an mRS score of 0–2 at 90–120 days (adjusted RR: 1.0 [95% CI 1.0 to 1.1]).
Actual mRS score at 90–120 days (n=1,567)
Actual mRS score at 90–120 days (n=1,567)
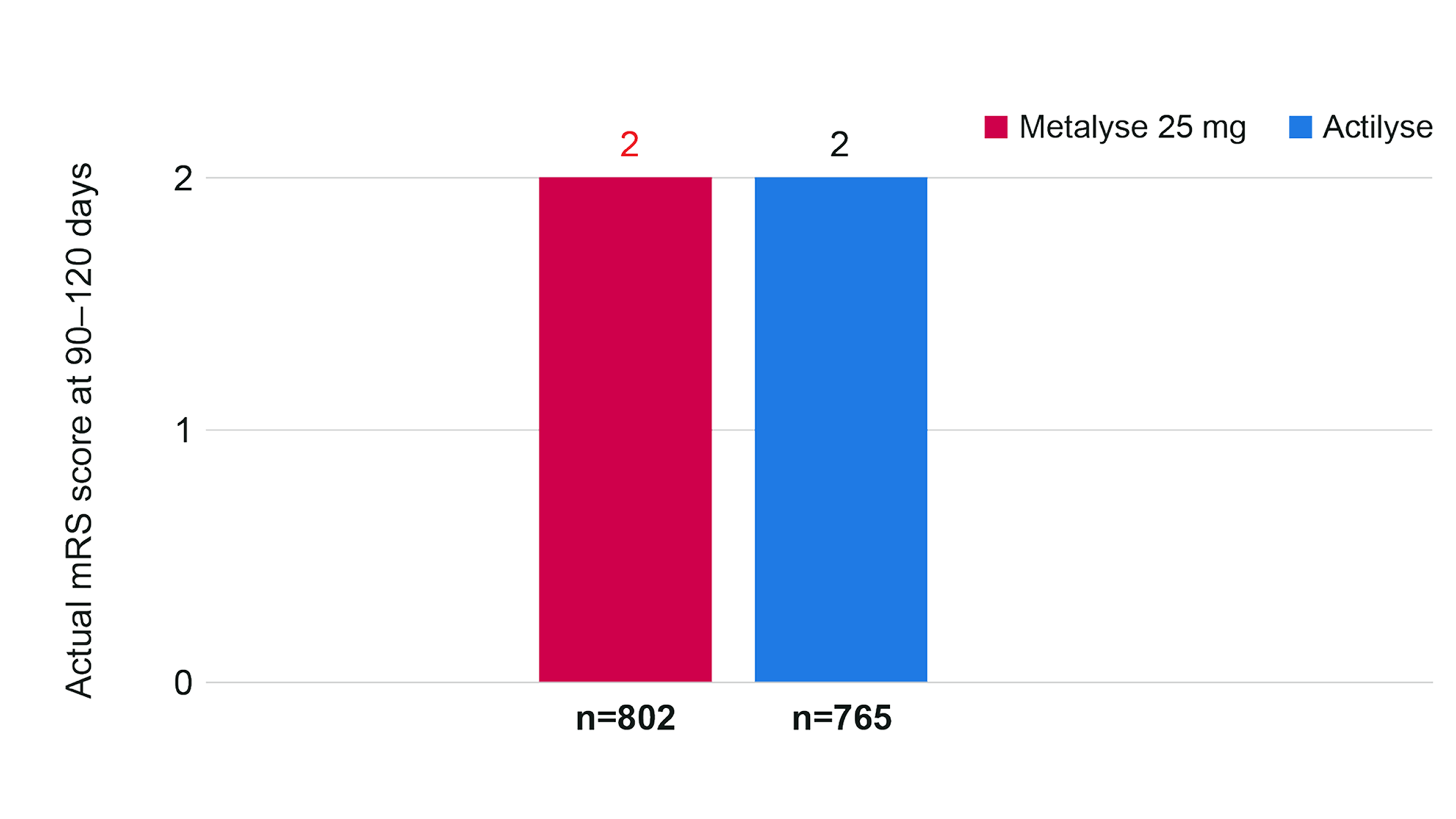
Adapted from Menon BK, et al. 2022.3
For both treatment arms, median (IQR) mRS was 2 (1–4) at 90–120 days, indicating comparable function between groups (adjusted common OR, 0.9 [95% CI 0.8 to 1.1]).
Safety: no meaningful differences in safety outcomes vs Actilyse†3
There were no meaningful differences in the rates of symptomatic intracerebral haemorrhage (sICH),‡ orolingual angio-oedema, extracranial bleeding requiring blood transfusion (all occurring within 24 hours of thrombolytic administration), and 90-day all-cause mortality.3
Key safety outcomes in patients who received any dose of Metalyse 25 mg or Actilyse†3
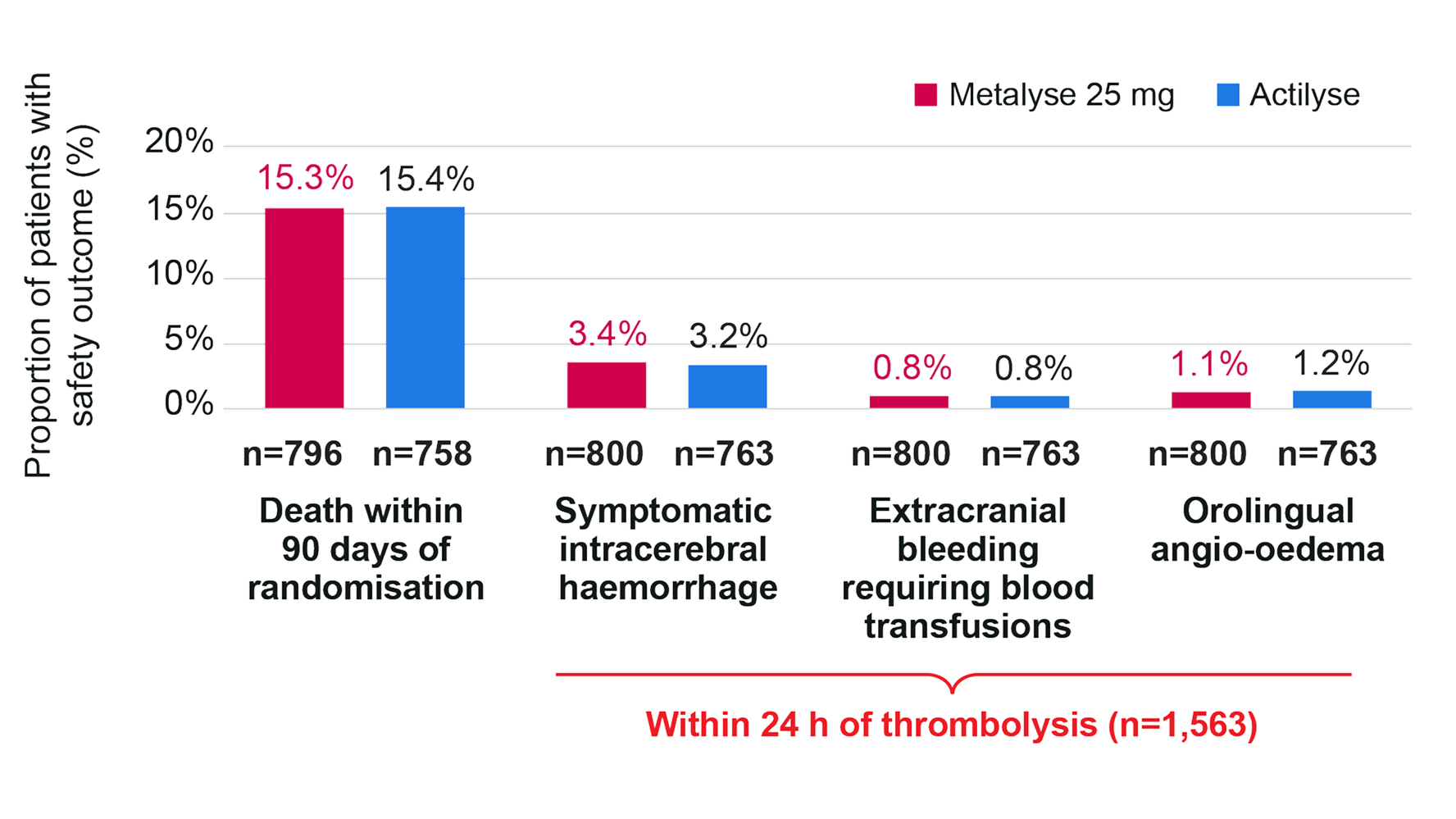
Adapted from Menon BK, et al. 2022.3
-
†
Safety population of 1,563 randomised patients who did not withdraw consent, and received Metalyse 25 mg or Actilyse (800 received Metalyse 25 mg, 763 received Actilyse).3
-
‡
24-hour sICH was defined as any ICH that was temporally related to, and directly responsible for, worsening of the patient’s neurological condition and in the investigator’s opinion was the most important factor for the neurological worsening.3
AcT study design
AcT was a phase III, investigator-initiated, multicentre, open-label, parallel-group, registry-linked, randomised controlled trial which investigated whether IV Metalyse 25 mg (decile-weight-based dosing of 0.25 mg/kg to a maximum of 25 mg; n=816. Refer to the SmPC for the full posology guidance) was non-inferior to IV Actilyse (0.9 mg/kg to a maximum of 90 mg; n=784) for the treatment of acute ischemic stroke in adults presenting with AIS within 4.5 hrs of symptom onset eligible for thrombolysis (per Canadian guidelines; study was conducted across Canada). The primary endpoint was the proportion of patients achieving a mRS score of 0–1 at 90–120 days. Key safety outcomes included 24-hour symptomatic intracerebral haemorrhage and 90-day all-cause mortality.3
ATTEST-2: Metalyse 25 mg demonstrated non-inferiority compared with Actilyse for distribution of functional outcomes (mRS score) at 90 days post-initiation4
The phase III ATTEST-2 trial compared the efficacy and safety of Metalyse 25 mg with Actilyse in adult UK patients with AIS presenting within 4.5 hours of onset and eligible for thrombolysis.4
Primary outcome: non-inferiority vs Actilyse for mRS score distribution at 90 days4
Metalyse 25 mg was non-inferior to Actilyse for the primary outcome but was not superior (OR: 1.07 [95% CI 0.90 to 1.27]; P < 0.0001 for non-inferiority; P = 0.43 for superiority).4
Distribution of the mRS scores at 90 days (n=1,663)4
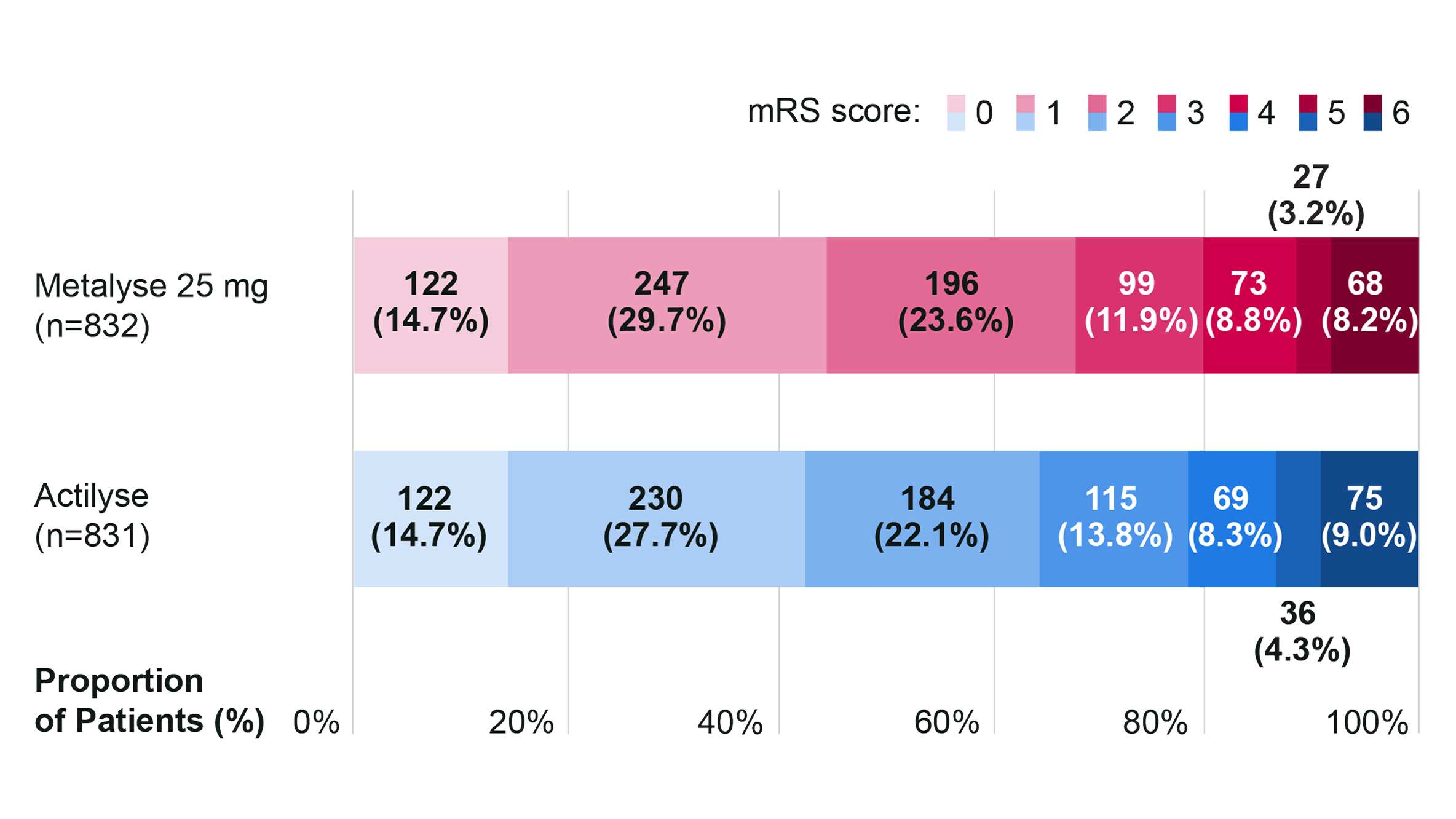
Adapted from Muir K, et al. 2024.4
mRS scores range from 0 to 6, with 0 indicating no symptoms, 1 no clinically significant disability, 2 slight disability, 3 moderate disability, 4 moderately severe disability, 5 severe disability, and 6 death.
Secondary outcomes: non-inferiority vs Actilyse for excellent neurological recovery4
The absolute difference in the proportion of patients with excellent neurological recovery (defined as mRS 0–1 vs 2–6) was 2.03% (95% CI −2.71 to 6.77).4
This met the predefined criteria for non-inferiority (P = 0.0018) but not for superiority (P = 0.40). The adjusted OR was 1.05 (95% CI 0.85 to 1.30; P = 0.66).4
Excellent neurological recovery (defined as mRS 0–1) at day 90 (n=1,663)
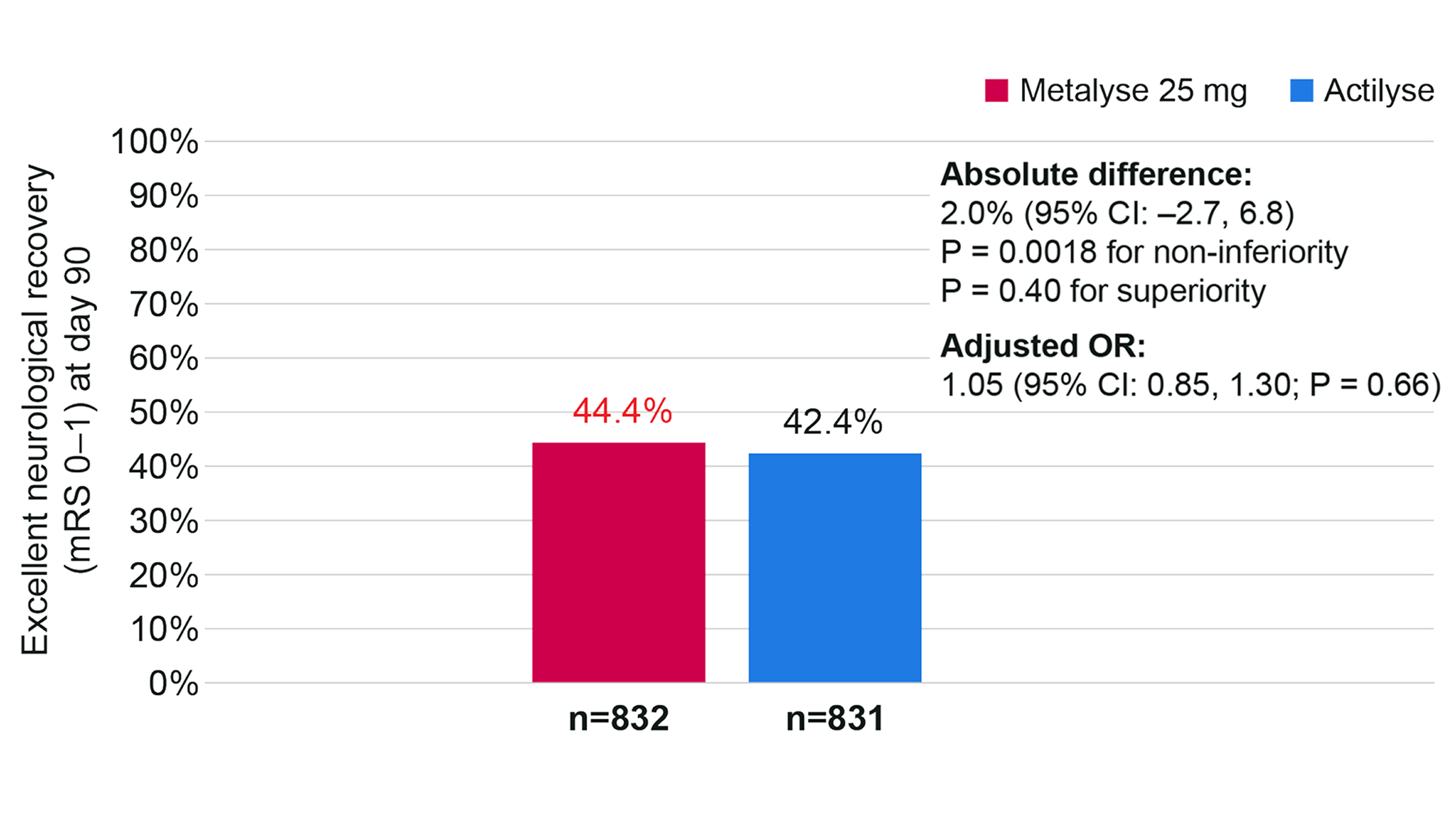
Adapted from Muir K, et al. 2024.4
Comparison of independent neurological recovery (mRS 0–2) at day 90
The absolute difference in the proportion of patients with independent neurological recovery (defined as mRS 0–2), was 3.41% (95% CI −1.14 to 7.95; P = 0.14; adjusted OR: 1.15 [95% CI 0.92 to 1.45]; P = 0.23) but there was no statistically significant difference.4
Independent neurological recovery (mRS 0–2) at day 90 (n=1,663)
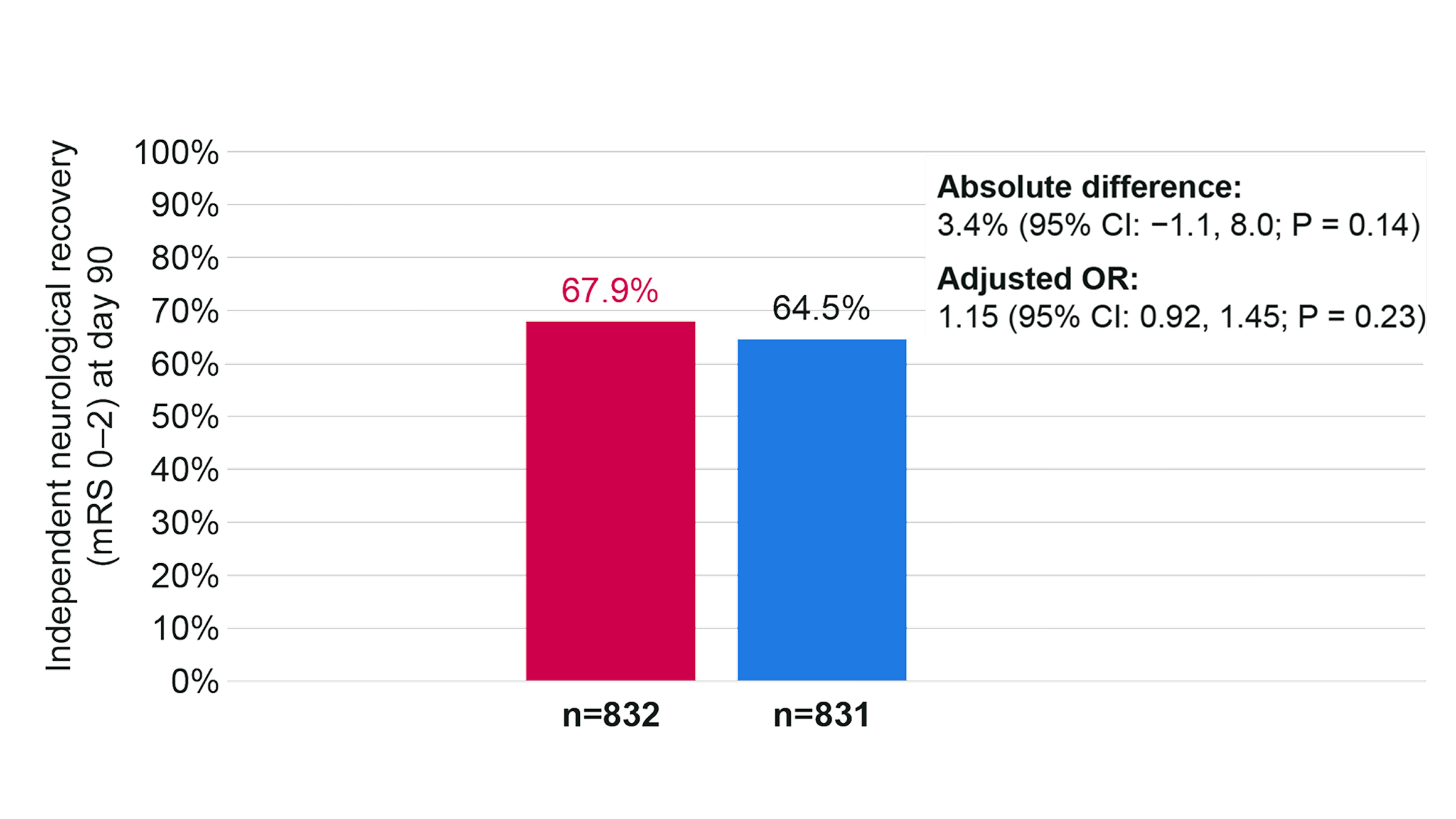
Adapted from Muir K, et al. 2024.4
Safety: no statistically significant differences in safety outcomes vs Actilyse4
There were no significant differences between treatment groups for safety outcomes.4
Death occurred for 8% of patients in the Metalyse 25 mg group (n/N=68/884) and 8% patients in the Actilyse group (n/N=75/893).4
Frequency of select safety outcomes in patients treated with Metalyse 25 mg or Actilyse§
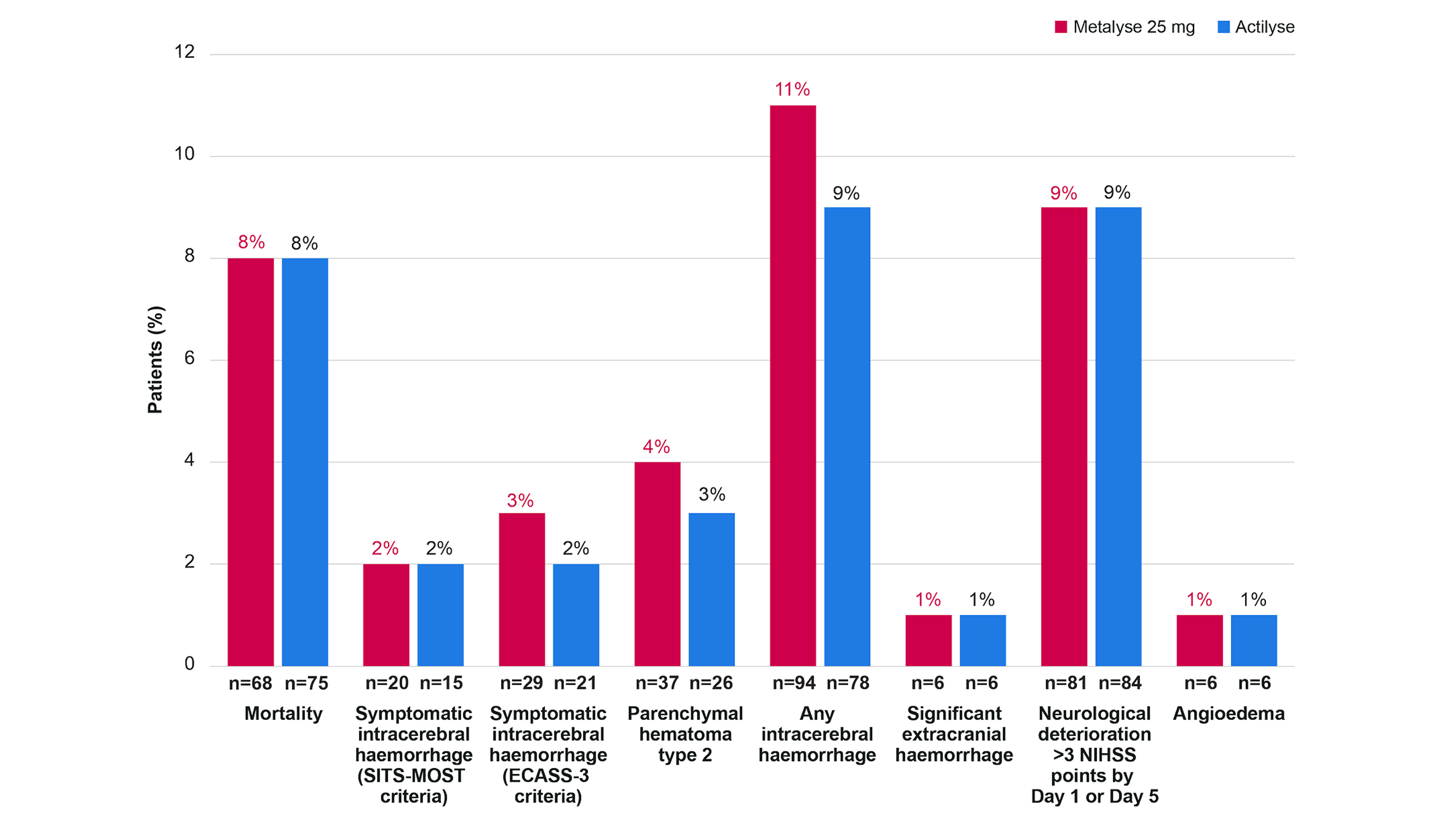
Adapted from Muir K, et al. 2024.4
All analyses are adjusted for the variables used in the randomisation minimisation (age group, stroke severity, and onset to randomisation time) and sex.
-
§
Safety was analysed in participants according to the treatment received (i.e., 884 received Metalyse 25 mg and 893 received Actilyse).
ATTEST-2 Study design
ATTEST-2 was a phase III, investigator-initiated, prospective, randomised, open-label, blinded endpoint trial which investigated whether IV Metalyse 25 mg (0.25 mg/kg to a maximum of 25 mg¶; n=885) was non-inferior to IV Actilyse (0.9 mg/kg to a maximum of 90 mg; n=892) for the treatment of acute ischemic stroke in adult UK patients presenting with AIS within 4.5 hrs of symptom onset eligible for thrombolysis (according to national clinical guidelines). The primary endpoint was distribution of mRS score at 90 days. Safety outcomes included mortality, symptomatic intracerebral haemorrhage, extracranial haemorrhage and angioedema.4
-
¶
The licensed posology for Metalyse 25 mg is based on tiered-weight-based dosing. Please refer to the SmPC or the dosing table above for the full recommended posology.
EXTEND-IA TNK: Metalyse 25 mg demonstrated higher incidence of reperfusion vs Actilyse in patients eligible for thrombectomy5
The phase II EXTEND-IA TNK trial compared IV Metalyse 25 mg with IV Actilyse in patients with LVO-AIS within 4.5 hours of onset who were eligible for mechanical thrombectomy.5
Primary outcome: non-inferiority, followed by superiority testing of reperfusion5
Treatment with Metalyse 25 mg was associated with significantly higher incidence of substantial reperfusion (reperfusion of >50% of the occluded vascular territory before thrombectomy or an absence of retrievable thrombus at the time of the initial angiographic assessment) vs Actilyse (incidence difference 12% [95% CI: 2% to 21%] P = 0.002 for noninferiority); adjusted incidence ratio, 2.2 [95% CI: 1.1 to 4.4]; P = 0.03 for superiority).ǁ
Primary efficacy outcome: substantial reperfusion at initial angiographic assessmentǁ
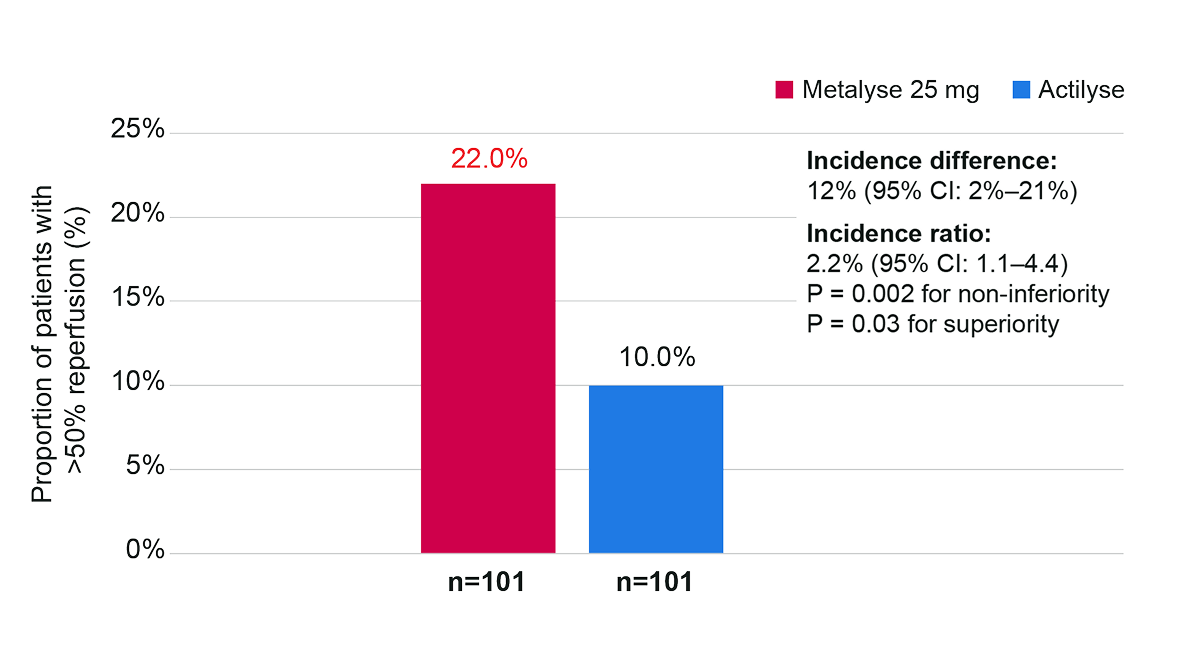
Adapted from Campbell BCV, et al. 2018.5
-
ǁ
Substantial reperfusion was defined as the restoration of blood flow to greater than 50% of the involved territory or no retrievable thrombus at the time of the initial angiographic assessment. Perfusion was assessed using the modified Treatment in Cerebral Ischemia (mTICI) classification (scores range from 0 [no flow] to 3 [normal flow]), which was adjusted for the site-of-vessel-occlusion strata. The P value for the difference is for noninferiority, and the P values for the incidence ratio and odds ratio are for superiority.5
Key secondary outcome
Metalyse 25 mg resulted in a better 90-day functional outcome** than Actilyse (median mRS score of 2 vs 3; common odds ratio, 1.7; 95% CI: 1.0 to 2.8; P = 0.04).5
At 90 days, 51% of patients in the Metalyse 25 mg group had a mRS score of 0 or 1, compared with 43% of patients in the Actilyse group (no significant difference; adjusted incidence ratio 1.2; 95% CI 0.9 to 1.6; P = 0.20).5
-
**
Functional outcome was measured as median mRS score at 90 days.5
Safety: no significant differences in safety outcomes vs Actilyse5
Death due to any cause: Metalyse 25 mg 10% (n=10/101) vs Actilyse 18% (n=18/101; P = 0.99), adjusted odds ratio 0.4 (95% CI 0.2 to 1.1; P = 0.08).5
Symptomatic intracerebral haemorrhage (defined as a large parenchymal haematoma [blood clot occupying >30% of the infarct volume with mass effect] and an increase of 4 points or more in the NIHSS score): Metalyse 25 mg 1% (n=1/101) vs Actilyse 1% (n=1/101; P = 0.99).5
No significant differences between Metalyse 25 mg and Actilyse in the rates of death by any cause5
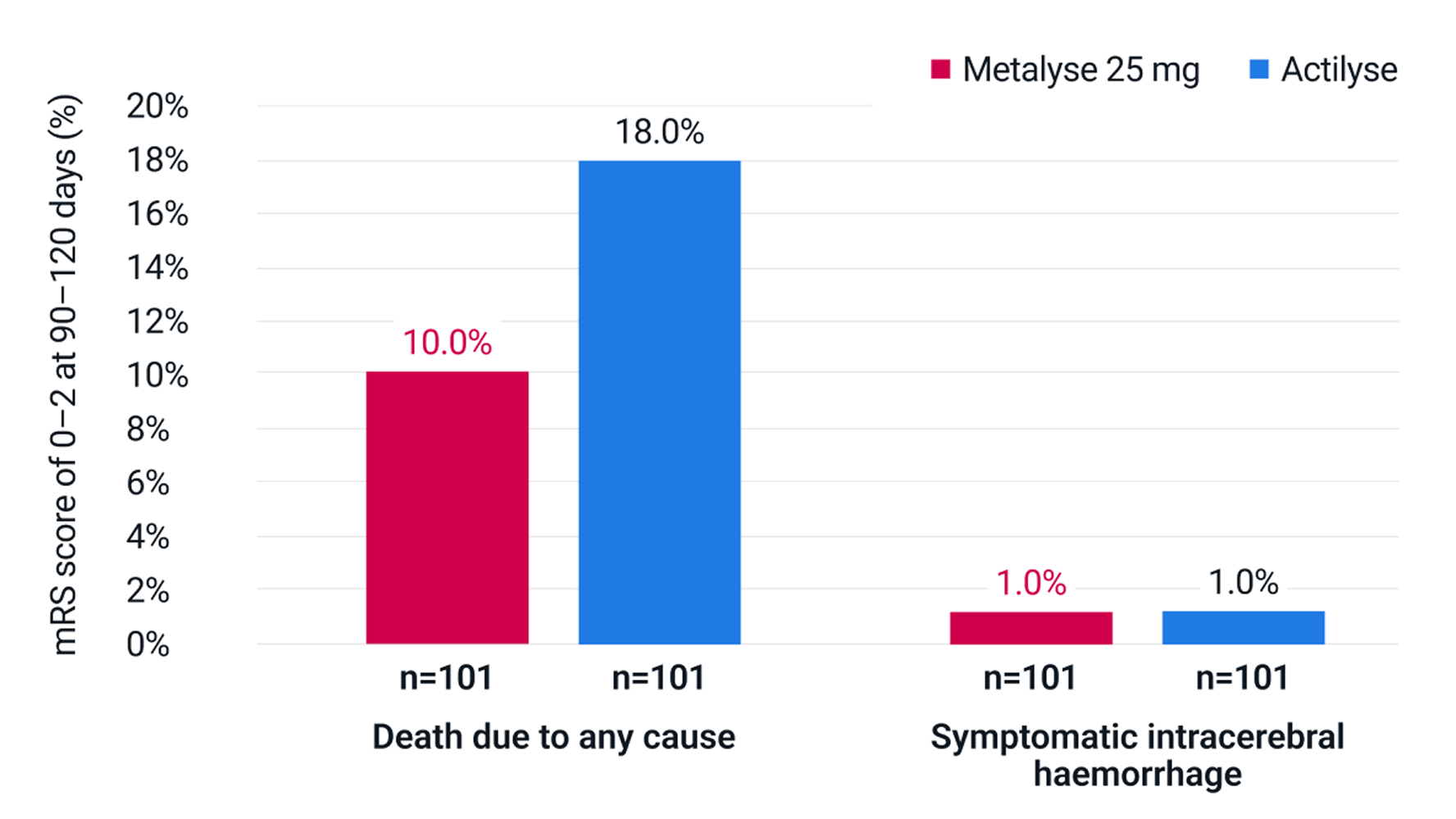
Adapted from Campbell BCV, et al. 2018.5
EXTEND-IA TNK study design
EXTEND-IA TNK was a phase II, investigator-initiated, prospective, multicentre, randomised, open-label, blinded-endpoint (PROBE) trial, which investigated whether Metalyse 25 mg (0.25 mg/kg to a maximum of 25 mg; n=101)†† was non-inferior to Actilyse (0.9 mg/kg to a maximum of 90 mg; n=101) in establishing reperfusion before thrombectomy in adult patients with LVO-AIS presenting within 4.5 hours of symptom onset eligible for thrombolysis and thrombectomy in Australia and New Zealand. The primary endpoint was the proportion of patients achieving substantial reperfusion (restoration of blood flow to greater than 50% of the involved territory or an absence of retrievable thrombus in the target vessel) at initial angiographic assessment. Key safety outcomes included symptomatic intracerebral haemorrhage and all-cause mortality.5
-
††
The licensed posology for Metalyse 25 mg is based on tiered-weight-based dosing. Please refer to the SmPC or the dosing table above for the full recommended posology.
Abbreviations
AIS: acute ischaemic stroke; CI: confidence interval; ICH: intracerebral haemorrhage; IQR: interquartile range; IV: intravenous; LVO: large vessel occlusion; mRS: modified Rankin Scale; mTICI: modified treatment in cerebral infarction; NIHSS: National Institutes of Health Stroke Scale; OR: odds ratio; RR: risk ratio; sICH: symptomatic intracerebral haemorrhage.
References
- Metalyse® 25 mg (tenecteplase) Summary of Product Characteristics.
- Actilyse® (alteplase) Summary of Product Characteristics.
- Menon BK, et al. Lancet. 2022;400:161–169.
- Muir K, et al. Lancet Neurol. 2024;23:1087–1096 (including supplementary appendix).
- Campbell BCV, et al. N Engl J Med. 2018;378:1573–1582 (including supplementary appendix).
- Boehringer Ingelheim. Data on File. MET 24-01.
- National Clinical Guideline for Stroke for the UK and Ireland. London: Intercollegiate Stroke Working Party; 2023 May 4. Available at: www.strokeguideline.org (accessed August 2025).
- Haley EC et al. Stroke. 2010;41:707–711.
- Huang X, et al. Int J Stroke. 2016;11:534–543.
- Parsons M, et al. N Engl J Med. 2012; 366: 1099–1107.
- Huang X, et al. Lancet Neurol 2015;14:368–376.
- Ranta A, et al. Eur Stroke J. 2023;8:942–946.
- Zitek T et al. West J Emerg Med. 2020;21:199–202.
PC-GB-110820 V2 August 2025