ThiNK Stroke
Stroke is a life-threatening medical emergency and can lead to life-threatening complications.2 The sooner it is treated, the greater the chances of neurological improvement and functional recovery.3 Explore below to learn how Metalyse 25 mg, a genetically modified version of Actilyse® (alteplase),1 could benefit patients with acute ischaemic stroke (AIS).
Disease burden

In the UK, stroke is responsible for deaths each year

Last year, 95,222 patients presented with stroke in England, Wales, and Northern Ireland. 10,803 Scottish residents had a final diagnosis of stroke

AIS accounts for ~85% of total stroke admissions in England, Wales, Northern Ireland and Scotland
Treatment pathway
The National Clinical Guideline for Stroke for the UK and Ireland state that intravenous (IV) thrombolysis with Actilyse or Metalyse 25 mg should be considered for patients with AIS within 4.5 hours of known onset.7
Metalyse 25 mg is clinically non-inferior to Actilyse for efficacy, with a comparable safety profile, and should be considered as an alternative fibrinolytic agent to the current standard of care.1,7–11
Importance of rapid intervention
The sooner treatment begins, the greater the average health benefits over a patient's lifetime.12 That's why when it comes to stroke, every minute matters.
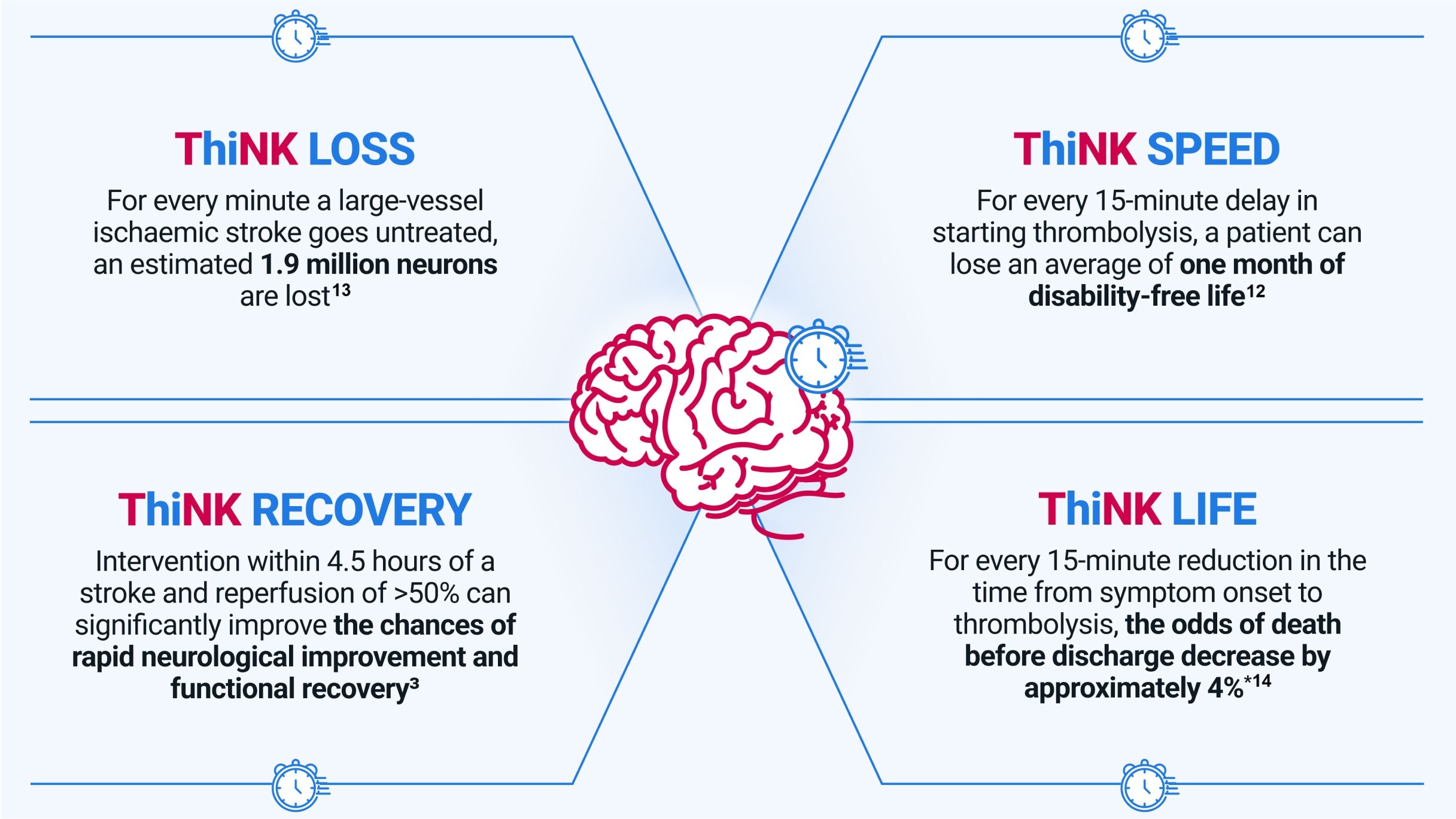
* The odds of death before discharge in a retrospective analysis of data from 58,353 patients with AIS decreased by approximately 4% with every 15-minute acceleration in start of treatment with tPA within 4.5 hours of symptom onset.14
ThiNK Thrombolysis: Metalyse 25 mg can save you precious minutes vs Actilyse
Metalyse 25 mg is indicated in adults for the thrombolytic treatment of AIS within 4.5 hours from last known well and after exclusion of intracranial haemorrhage.1
Metalyse 25 mg has non-inferior efficacy and comparable safety to Actilyse, delivering the same high standard of outcomes that healthcare professionals are familiar with.1,8-11
With a single IV bolus and no requirement for an IV infusion pump, Metalyse 25 mg is simpler and quicker to administer than Actilyse.1,11
In a real-world setting, tenecteplase was associated with a faster door-to-needle (DTN) time than Actilyse in the treatment of AIS (median DTN [IQR]: 52 [47–83] minutes vs 61 [45–84] minutes, respectively; p<0.0001).†15
These key differences mean that switching from Actilyse to Metalyse 25 mg could save precious minutes for your patient, potentially improving their chances of recovery.15
Metalyse 25 mg can save up to 60 minutes with every patient because of its simpler and quicker administration than Actilyse. During a 12-month period, Metalyse 25 mg could save up to 461 days and 19 hours based on 11,083 patients thrombolysed (Apr 2023–Mar 2024).16
† An analysis of real-world data for 1,408 patients from a compulsory national stroke reperfusion register in the Central Region of New Zealand. Data were extracted for all adult patients treated with tenecteplase 0.25mg/kg or alteplase 0.9 mg/kg between 1 January 2018 and 31 December 2022.15


Abbreviations
AIS: acute ischaemic stroke, DTN: door-to-needle, IQR: interquartile range, IV: intravenous, tPA: tissue-type plasminogen activator.
References
- Metalyse® 25 mg (tenecteplase) Summary of Product Characteristics.
- NHS. Stroke. Available at: www.nhs.uk/conditions/stroke/ (accessed July 2025).
- Emberson J, et al. Lancet. 2014;384:1929–1935.
- NICE. Stroke and TIA: What is the prevalence of stroke and TIA in the UK? Available at: cks.nice.org.uk/topics/stroke-tia/background-information/prevalence/ (accessed July 2025).
- Sentinel Stroke National Audit Programme. Annual Report 2024. Available at: www.strokeaudit.org/Results2/Clinical-audit/National-Results.aspx (accessed July 2025).
- Public Health Scotland. Scottish Stroke Improvement Programme 2024, with data from the Scottish Stroke Care Audit: national report. Available at: https://publichealthscotland.scot/media/28142/scottish-stroke-improvement-programme-annual-report-2024-final-v-10.pdf (accessed July 2025).
- National Clinical Guideline for Stroke for the UK and Ireland. London: Intercollegiate Stroke Working Party; 2023 May 4. Available at: www.strokeguideline.org (accessed July 2025).
- Menon BK, et al. Lancet. 2022;400:161–169.
- Campbell BCV, et al. N Engl J Med. 2018;378:1573–1582.
- Muir K, et al. Lancet Neurol. 2024;23:1087–1096
- Actilyse® (alteplase) Summary of Product Characteristics.
- Meretoja A, et al. Stroke. 2014;45:1053–1058.
- Saver JL, Stroke. 2006;37:263–266.
- Saver JL, et al. JAMA. 2013;309:2480–2488.
- Ranta A, et al. Eur Stroke J. 2023;8:942–946.
- Sentinel Stroke National Audit Programme. Annual results portfolio, Apr 2023 – Mar 2024. Available at: https://www.strokeaudit.org/Results2/Clinical-audit/National-Results.aspx (accessed July 2025).
PC-GB-110818 V2 July 2025


