Dosing & Administration
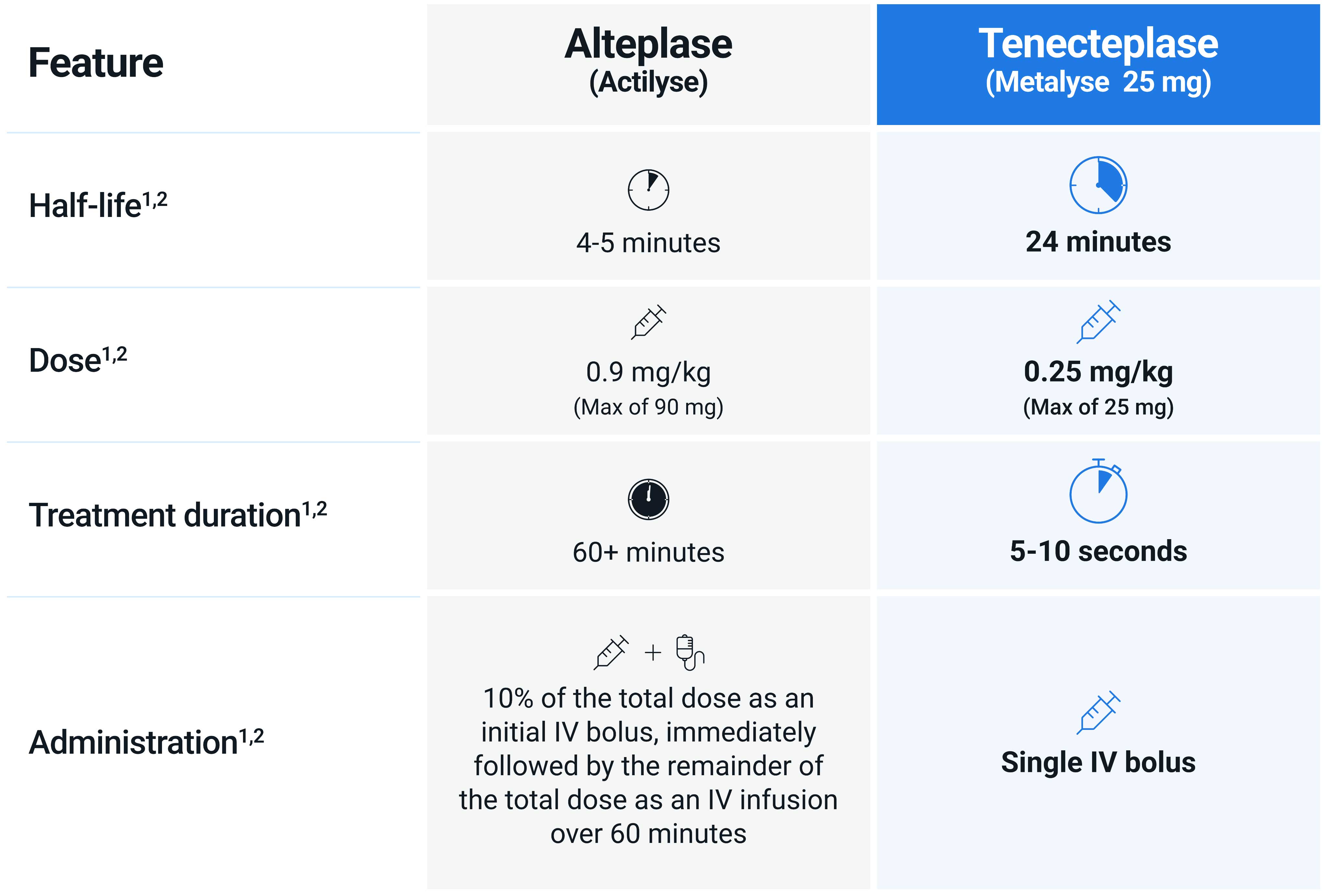
With five weight-based tiers, a single IV bolus administered over approximately 5–10 seconds, and no requirement for an IV infusion pump, Metalyse 25 mg is simpler and quicker to administer than Actilyse® (alteplase).1,2
Metalyse 25 mg is fast and easy to administer
Metalyse 25 mg does not require an infusion pump, greatly simplifying administration, when compared to Actilyse.1,2
As Metalyse 25 mg is administered as a single IV bolus, it does not have the delay between the bolus and infusion stages associated with Actilyse. This delay may potentially interfere with the efficacy of Actilyse.3,4
Metalyse 25 mg is incompatible with glucose infusion solutions.1
Why is Metalyse 25 mg faster and easier to use than Actilyse?
Tenecteplase has different pharmacokinetic and pharmacodynamic properties compared with alteplase, facilitating faster and easier administration.1,2,5,6
The longer half-life of tenecteplase allows for a single 5–10 second bolus administration.1,2,5,6
How to Reconstitute Metalyse 25 mg
Prior to reconstitution, please ensure to source your syringe, needle, and sterile water for injection, from your hospital.

Metalyse 25 mg reconstitution guide
Metalyse 25 mg reconstitution video
Dosing table
The Metalyse 25 mg dose of 0.25mg/kg was identified through Phase II trials as the most appropriate when considering efficacy and safety data. The licensed posology, as used in the AcT trial, used decile-weight-tiered dosing seen in the table below.7-11
Metalyse 25 mg should be administered on the basis of body weight, with a maximum single dose of 5,000 units
(25 mg tenecteplase) for acute ischaemic stroke. Benefit-risk of Metalyse 25 mg treatment should be carefully evaluated in patients weighing 50 kg or less due to limited availability of data.1
| Patient's body weight (kg) | Tenecteplase (U) | Tenecteplase (mg) | Corresponding volume of reconstituted solution (ml) |
|---|---|---|---|
| <60 | 3,000 | 15.0 | 3.0 |
| ≥60 to <70 | 3,500 | 17.5 | 3.5 |
| ≥70 to <80 | 4,000 | 20.0 | 4.0 |
| ≥80 to <90 | 4,500 | 22.5 | 4.5 |
| ≥90 | 5,000 | 25.0 | 5.0 |
Treatment with Metalyse 25 mg must be initiated as early as possible after onset of symptoms, and no later than 4.5 hours after last known well and after exclusion of intracranial haemorrhage by appropriate imaging techniques. The treatment effect is time-dependent; therefore, earlier treatment increases the probability of a favourable outcome.
Metalyse 25 mg should be prescribed by physicians experienced in the use of thrombolytic treatment and with the facilities to monitor that use.
Thrombolytic therapy is associated with a risk of bleeding. Thrombolysis in AIS patients should be evaluated on individual benefit-risk basis. Metalyse 25 mg should be administered with caution in the elderly (>80 years) due to a higher bleeding risk.
For further details of factors which may be associated with an increased risk of bleeding, and situations in which treatment is contraindicated, please refer to the Summary of Product Characteristics or see our page on Contraindications.
Medicinal products that affect coagulation or those that alter platelet function may increase the risk of bleeding prior to, during or after tenecteplase therapy and should be avoided in the first 24 hours after treatment for acute ischaemic stroke.
Prior to prescribing Metalyse 25 mg, please refer to the Summary of Product Characteristics for full information on contraindications, special warnings and precautions for use.
Abbreviations
AIS: acute ischaemic stroke, IV: intravenous.
References
- Metalyse® 25 mg (tenecteplase) Summary of Product Characteristics.
- Actilyse® (alteplase) Summary of Product Characteristics.
- Kobayashi Y, et al. J Emerg Med 2023;64(6);709-713.
- Acheampong P, et al. Stroke Res Treat 2014; doi:10.1155/2014/358640.
- Baird AE, et al. Semin Neurol. 2021;41:28–38.
- Keyt BA, et al. Proc Natl Acad Sci USA. 1994;91:3670–3674.
- Campbell BCV, et al. N Engl J Med. 2018;378:1573–1582.
- Haley EC et al. Stroke. 2010;41:707–11.
- Huang X, et al. Int J Stroke. 2016;11:534–43.
- Parsons M, et al. N Engl J Med. 2012;366:1099–107.
- Huang X, et al. Lancet Neurol 2015;14:368–76.
- Ranta A, et al. Eur Stroke J. 2023;8:942–946.
PC-GB-110819 V3 August 2025