Mechanism of Action
How does Trajenta® work?
Trajenta® is a ‘gliptin’ and an inhibitor of the enzyme DPP-4 (dipeptidyl peptidase-4), which is involved in the inactivation of the incretin hormones GLP-1 (glucagon-like peptide-1) and GIP (glucose-dependent insulinotropic polypeptide). Both incretin hormones are involved in the physiological regulation of glucose homeostasis and these hormones are rapidly degraded by the enzyme DPP-4.1 Trajenta® selectively inhibits DPP-4, which leads to a glucose-dependent increase in insulin secretion and a reduction in glucagon secretion thus resulting in an overall improvement in glucose homeostasis.1 DPP-4 inhibitors prolong the action of GLP-1 and GIP by working in a glucose-dependent way.2,3


Trajenta® is excreted primarily via the bile.
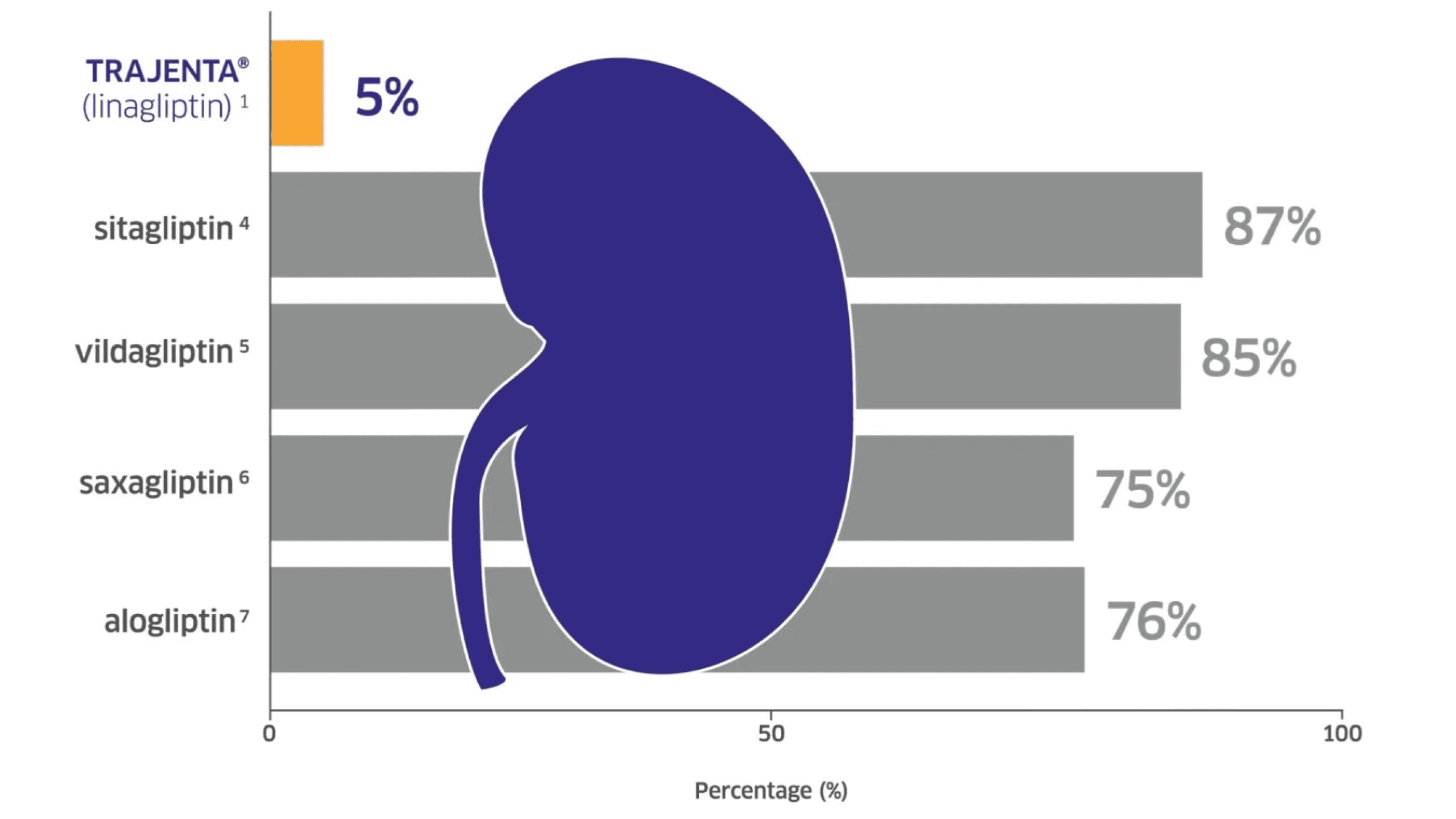
Only 5% of Trajenta® is excreted through the kidney and it can therefore be used in patients at any stage of renal function.1
Proportions of medication excreted via the kidney
Please refer to the individual product SmPC for full information. Renal monitoring should be undertaken as per NICE guidelines.
Simple dosing and administration

Proven safety and efficacy via clinical studies
Abbreviations:
3P-MACE: 3-point major adverse cardiac events; CV: cardiovascular; CVOT: cardiovascular outcome trial; MI: myocardial infarction; T2D: type 2 diabetes.
Footnotes
-
*
CARMELINA® and CAROLINA® included 6,979 and 6,033 patients respectively.8,11 Primary endpoint for these trials: Time to first occurrence of any of the following CV components: CV death (including fatal stroke and fatal MI), non-fatal MI (excluding silent MI), or non-fatal stroke (3P-MACE).8,11
-
†
Sulphonylureas and insulin are known to cause hypoglycaemia. Therefore, caution is advised when linagliptin is used in combination with a sulphonylurea and/or insulin. A dose reduction of the sulphonylurea or insulin may be considered (Trajenta® SmPC).
References
- Trajenta® (linagliptin) Summary of Product Characteristics. SmPCs available at EMC.
- Drucker DJ. Expert Opin Invest Drugs 2003;12:87-100.
- Ahrén B. Curr Diab Rep 2003;3:365-372.
- Sitagliptin Summary of Product Characteristics. SmPCs available at EMC.
- Vildagliptin Summary of Product Characteristics. SmPC available at EMC.
- Saxagliptin Summary of Product Characteristics. SmPCs available at EMC.
- Alogliptin Summary of Product Characteristics. SmPCs available at EMC.
- Rosenstock J et al. JAMA 2019;321(1):69–79.
- Rosenstock J, et al. Cardiovasc Diabetol. 2018;17:39.
- Marx N, et al. Diab Vasc Res. 2015;12:164–74.
- Rosenstock J, et al. JAMA. 2019;322(12):1155–1166.
- Del Prato S, et al. J Diab Compl. 2013; 27:274-9.
Trajenta is indicated in adults with type 2 diabetes mellitus as an adjunct to diet and exercise to improve glycaemic control as:
monotherapy when metformin is inappropriate due to intolerance, or contraindicated due to renal impairment
in combination with other medicinal products for the treatment of diabetes, including insulin, when these do not provide adequate glycaemic control
PC-GB-110496 V2 | February 2025