Clinical Studies
Trajenta® is the only DPP-4 (dipeptidyl peptidase-4) inhibitor with two cardiovascular outcome trials (CVOTs). 13,000 type 2 diabetes (T2D) adult patients were included in CARMELINA® and CAROLINA®1-6,*
Cardiovascular outcome trials programme
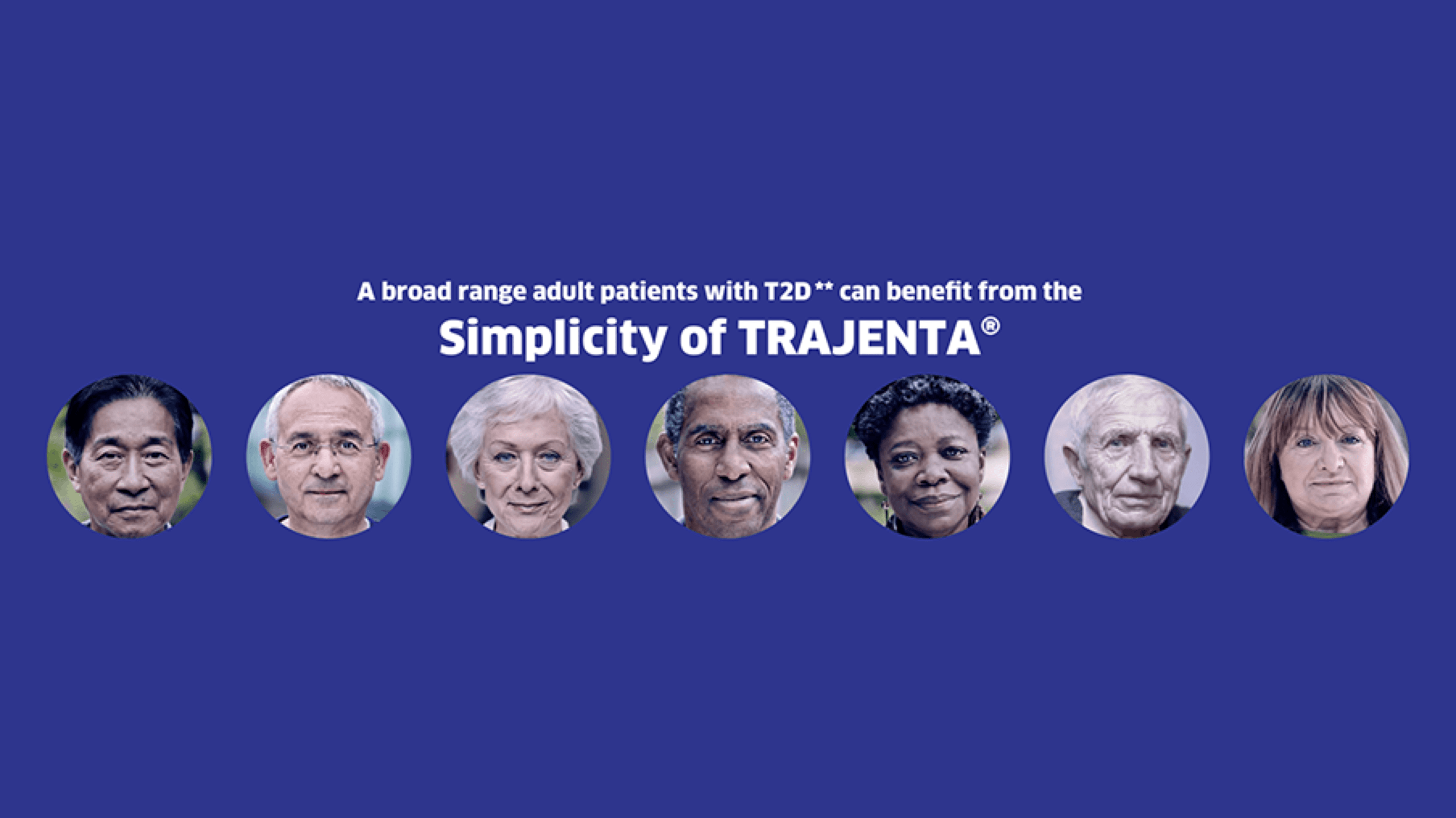
CV safety is an important concern when managing adult patients with T2D.1-4* Trajenta® demonstrated a safety profile across a broad range of T2D patients in two CVOTs including over 13,000 patients.1-4*
CARMELINA® and CAROLINA® constitute a robust* CVOT programme assessing the CV safety profile of Trajenta® in a broad range of adult T2D patients.1-4

Broad range of T2D patients
HR: 1.02 (95% CI 0.89, 1.17): p=0.74 for superiority p<0.001 for non-inferiority
HR: 0.98 (95% CI 0.84, 1.14); p=0.76 for superiority p<0.001 for non-inferiority

HR: 0.90 (95% CI 0.74, 1.08); p=0.26 for superiority

Incidence of ≥1 episode of hypoglycaemic event was lower with linagliptin (n=320 (10.6%)) vs. glimepiride (n=1132 (37.7%)) across all predefined hypoglycaemia-severity categories HR: 0.23 (95% CI, 0.21, 0.26)4
HR: 1.04 (95% CI 0.89, 1.22); p=0.62
Real world evidence
The dosing for Trajenta® is independent of renal function.7‡‡
A cross sectional study in 17,012 T2D patients treated with a DPP-4 inhibitor (DPP-4i) assessed the extent to which SmPC-recommended renal thresholds were adhered to in routine clinical practice.8 This study demonstrated that 33.6% (336/1000) treated with a non-linagliptin DPP-4i requiring dose reduction based on renal function received a higher dose than specified in the SmPC.8 Additionally, for patients who did not require a dose reduction 11.4% (1,296/11,411) received a lower dose than specified in the SmPC.7,8##§§
Since linagliptin patients always receive the recommended dose, the results shown are from patients receiving one of the other four DPP-4i therapies included in the study.
Study designs:
Cohort 1: Patients expected to have their dose adjusted according to their level of renal function (n=1,000): included patients with index prescriptions for: saxagliptin or sitagliptin (+ eGFR <45 ml/min), alogliptin (+ CrCl ≤50 ml/min) or vildagliptin (+ CrCl <50 ml/min).8
Cohort 2: Patients expected to receive the full SmPC recommended dose (n=11,411) including patients with index prescriptions for: saxagliptin or sitagliptin (+ eGFR ≥45 ml/min), alogliptin (+ CrCl >50 ml/min) or vildagliptin (+ CrCl ≥50 ml/min).8

Trajenta® is excreted primarily via the bile

Demonstrated safety profile
Footnotes
-
*
CARMELINA® and CAROLINA® included 6,979 and 6,033 patients respectively.
-
†
CARMELINA® included patients with albuminuria & previous macrovascular disease, and/or impaired kidney function with or without CV comorbidities.
-
‡
When added to standard of care.
-
#
The CARMELINA® primary endpoint was time to first occurrence of any of the following components: cardiovascular (CV) death, non-fatal myocardial infarction (MI), non-fatal stroke. The primary endpoint occurred in 434/3,494 (12.4%) and 420/3,485 (12.1%) patients in the linagliptin and placebo groups, respectively (HR: 1.02 (95% CI, 0.89, 1.17) non-inferiority p<0.001).
-
§
The CARMELINA® key secondary endpoint was time to first occurrence of any of the following components: Death due to kidney disease, sustained end-stage renal disease (ESRD) or a sustained decrease of ≥40% in estimated glomerular filtration rate (eGFR) from baseline. The key secondary kidney endpoint occurred in 327/3,494 (9.4%) and 306/3,485 (8.8%) patients in the linagliptin and placebo groups, respectively (HR: 1.04 (95% CI, 0.89, 1.22) p=0.62).
-
††
Because the test for superiority of the primary endpoint was null, findings for the secondary outcomes should be interpreted as exploratory. Time to first occurrence of any hypoglycaemic adverse event within the treated set (events occurring between first study drug intake until 7 days after last permanent study drug stop). Percentage of patients experiencing a hypoglycaemic event was 10.6% for linagliptin and 37.7% for glimepiride (HR: 0.23 (95% CI, 0.21, 0.26) non-inferiority p<0.001).
-
‡‡
Renal and other monitoring in patients with type 2 diabetes should be undertaken as per National Institute for Health and Care Excellence (NICE) Guidelines (NG28 T2DM and NG203 CKD).9,10
-
##
Patients with Type 2 Diabetes aged ≥18 years, treated with DPP-4 inhibitors from 15 July 2018 were identified in the Clinical Practice Research Datalink (CPRD) Database. Patients on linagliptin were excluded from the denominator for percentage calculations as no dose reduction is required regardless of renal function.
-
§§
For individual dosing on DPP-4 inhibitor dosing in renal impairment, please refer to the individual product SmPC.
-
†††
The CAROLINA® primary endpoint was time to first occurrence of any of the following components: CV death, non-fatal myocardial infarction, or nonfatal stroke (3-P MACE). 3-P MACE outcomes did not differ between linagliptin and glimepiride overall (HR: 0.98 (95.47% CI, 0.84, 1.14) or across age groups (interaction P>0.05)).
Abbreviations:
CI: confidence interval; CrCl: creatinine clearance; CV: cardiovascular; CVOT: cardiovascular outcome trial; DPP-4: dipeptidyl peptidase-4; DPP-4i: dipeptidyl peptidase-4 inhibitor; eGFR: estimated glomerular filtration rate; HHF: hospitalization for heart failure; HR: hazard ratio; SmPC: Summary of Product Characteristics; T2D: type 2 diabetes.
References
- Rosenstock J et al. JAMA 2019;321(1):69–79.
- Rosenstock J, et al. Cardiovasc Diabetol. 2018;17:39.
- Marx N, et al. Diab Vasc Res. 2015;12:164–74.
- Rosenstock J, et al. JAMA. 2019;322(12):1155–1166.
- Cooper M, et al. Diabetes Obes Metab. 2020; 1–12.
- Espeland MA, et al. Diab Obes Met 2020. doi: 10.1111/dom.14254.
- Trajenta® (linagliptin) Summary of Product Characteristics. SmPCs available at EMC.
- Spanopoulos D, et al. Clin Ther 2019;41(18):1622–1630.
- NICE guidance NG203 November 2021. Available at: https://www.nice.org.uk/guidance/NG203 (last accessed November 2024).
- NICE guidance NG28 February 2022. Available at: https://www.nice.org.uk/guidance/NG28 (last accessed November 2024).
- Cid Ruzafa J, et al. Int J Clin Pract 2015. 69(8):871–882.
- Del Prato S, et al. J Diab Compl. 2013; 27:274-9.
Trajenta is indicated in adults with type 2 diabetes mellitus as an adjunct to diet and exercise to improve glycaemic control as:
monotherapy when metformin is inappropriate due to intolerance, or contraindicated due to renal impairment
in combination with other medicinal products for the treatment of diabetes, including insulin, when these do not provide adequate glycaemic control
PC-GB-110499 | December 2024