Efficacy
Clinical trials demonstrate that Trajenta® significantly improves glycaemic control1,2 regardless of renal function,2,3 or patient age.4
In phase III clinical trials, Trajenta® produced clinically significant improvements in glycaemic control.1,2
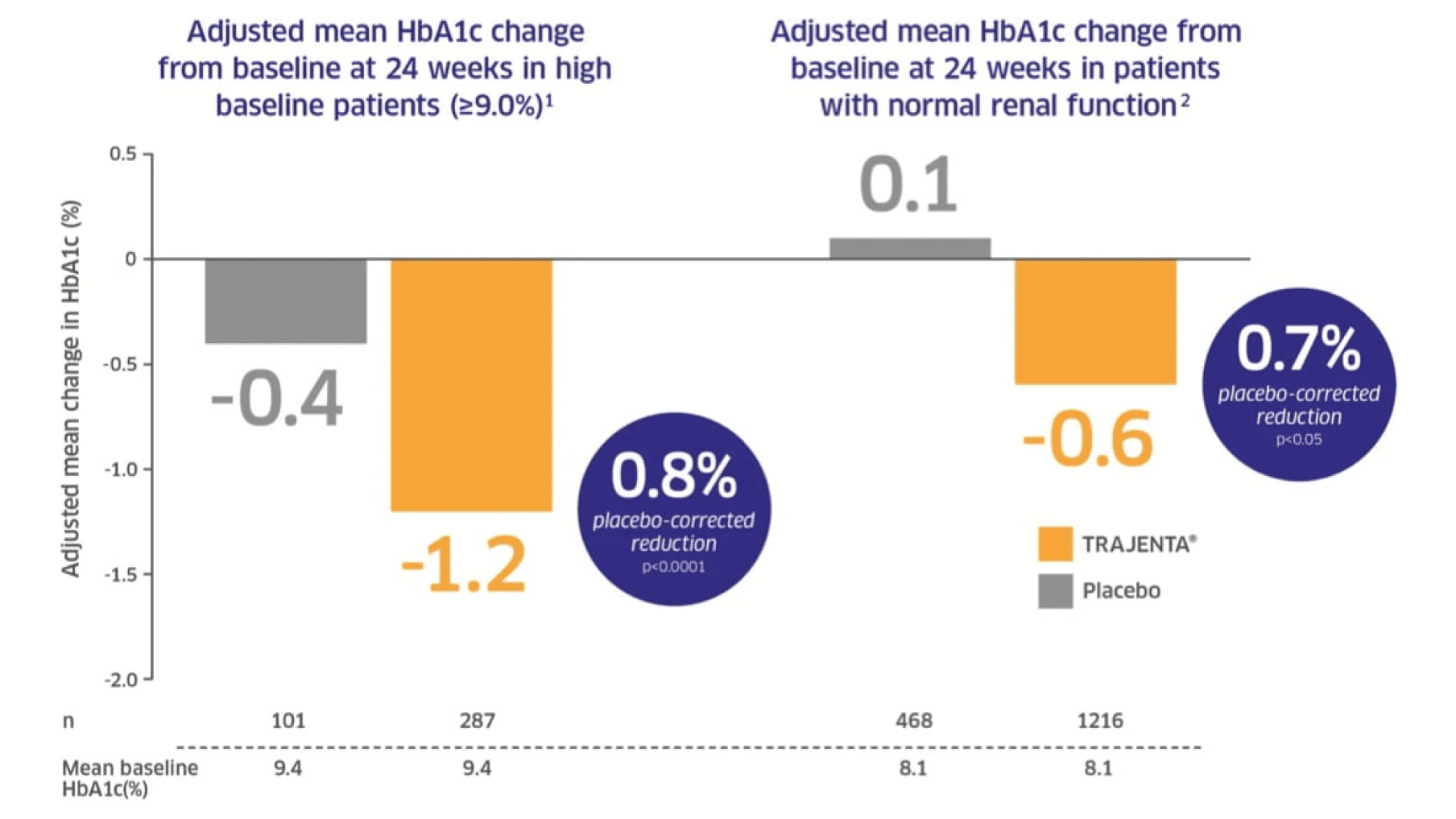
Adapted from: 1. Del Prato S, et al. J Diab Compl. 2013 2. Groop P-H, et al. Diabetes, Obesity and Metabolism. 2014.
Del Prato: Pooled analysis of data from 2,258 subjects in three, 24-week phase III, randomised, placebo-controlled, parallel-group studies, who received oral Trajenta® (5 mg/day) or placebo as monotherapy, added-on to metformin, or added-on to metformin plus sulphonylurea was performed. Results shown are from 388 patients who had a high mean baseline HbA1c of 79.2 mmol/mol (9.4%).1
Groop: Pooled analysis based on CrCl* data from 2,143 patients in three 24-week phase III trials. Change in baseline HbA1c between Trajenta® 5 mg and placebo was compared between three renal function groups.2
In randomised controlled trials (including three phase III trials), Trajenta® produced clinically significant improvements in glycaemic control regardless of renal function.2,3
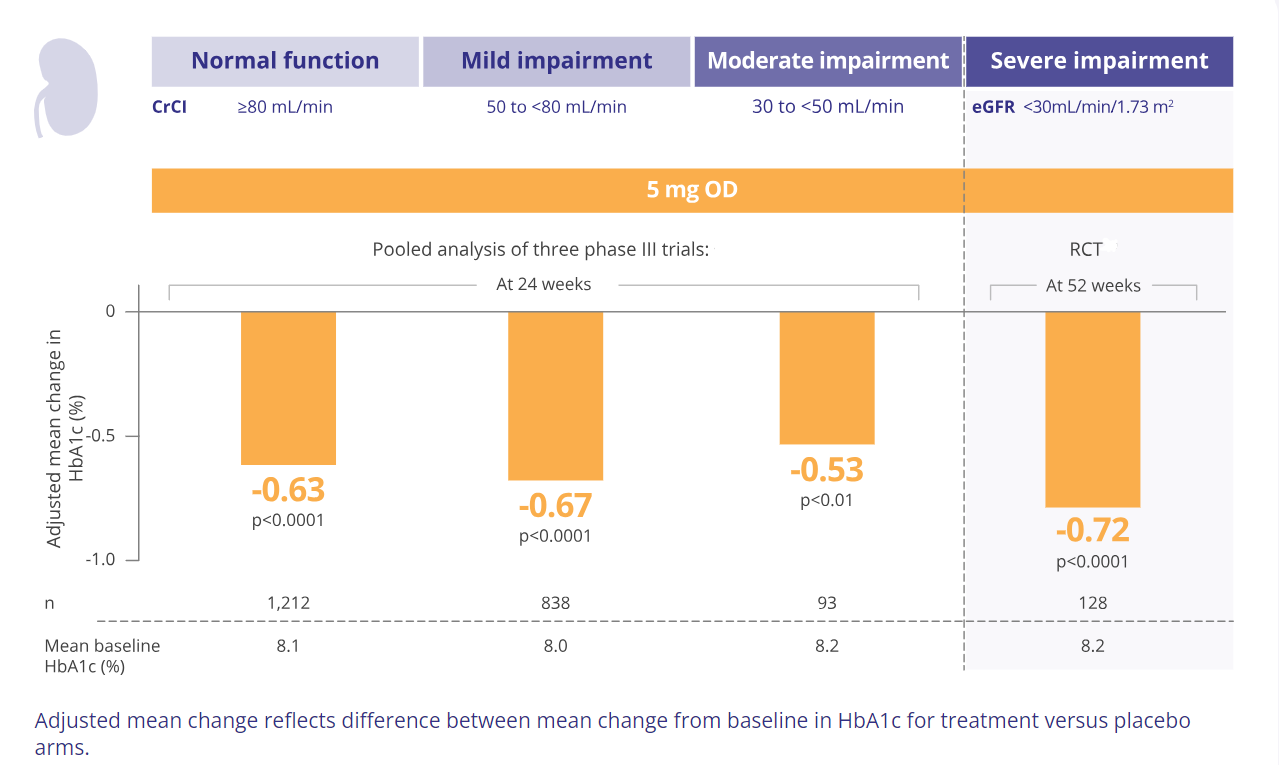
Adapted from: 2. Groop P-H, et al. Diabetes, Obesity and Metabolism. 2014 3. McGill JB, et al. Diabetes Care. 2013 5. Trajenta® SmPC.
Groop: Pooled analysis based on CrCl* data from 2,143 patients in three 24-week phase III trials. Change in baseline HbA1c between Trajenta® 5 mg and placebo was compared between three renal function groups.2
McGill: 1-year randomised, double blind, placebo-controlled study where treatment was added to existing background therapy. 133 patients (mean age 64.4 years) had severe renal impairment (eGFR <30 ml/min /1.73m2), an HbA1c of >7 and <10% and a BMI ≤45 kg/m2. 63.9% and 18% of the overall study population were treated with insulin alone and insulin combination respectively. Background therapy was fixed for the first 12 weeks. Between weeks 12–52, therapy could be adjusted according to glucose parameters. Primary endpoint: Change in HbA1c from baseline after 12 weeks.3
In phase III clinical trials, Trajenta® produced clinically significant improvements in glycaemic control.1,2
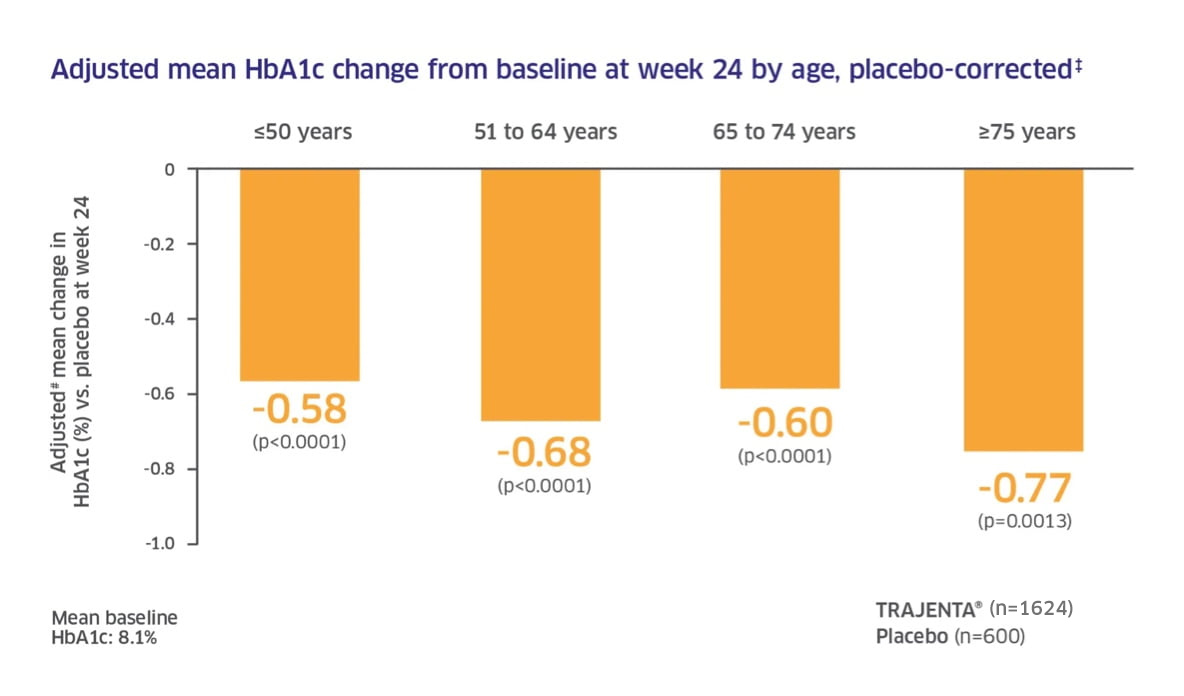
Adapted from: 4. Patel S, et al. European Association for the Study of Diabetes 2011, Poster P832
Patel: Pre–specified subgroup analysis on pooled data from three phase III, randomised, placebo–controlled trials: treatment in monotherapy, add-on to metformin, and add–on to metformin plus sulphonylurea. Primary endpoint: Change in HbA1c from baseline after 24 weeks (in all three studies).4
Robust clinical trials with thousands of patients

Demonstrated safety profile
Abbreviations:
3P-MACE: 3-point major adverse cardiac events; BMI: body mass index; CrCl: creatinine clearance; eGFR: estimated glomerular filtration rate; MI: myocardial infarction; SCr: serum creatinine; SmPC: Summary of Product Characteristics.
Footnotes
-
*
Creatine clearance = (140 - age) x body weight)/(72 x SCr), x 0.85 for females.
-
‡
ANCOVA-adjusted for continuous HbA1c, BMI group, washout phase, treatment group, study, age group, sex, time since diagnosis of diabetes, race and age x treatment or type 2 diabetes x treatment interactions.
-
#
p–values for between-group differences (vs. placebo).
-
**
CARMELINA® and CAROLINA® included 6,979 and 6,033 patients respectively.6,7 Primary endpoint for these trials: Time to first occurrence of any of the following CV components: CV death (including fatal stroke and fatal MI), non-fatal MI (excluding silent MI), or non-fatal stroke (3P-MACE).7
References
- Del Prato S, et al. J Diab Compl. 2013;27:274–9.
- Groop P-H, et al. Diabetes, Obesity and Metabolism. 2014:16:560-8.
- McGill JB, et al. Diabetes Care. 2013;36:237-44.
- Patel S, et al. European Association for the Study of Diabetes 2011, 12-16 September 2011, Lisbon, Portugal; Poster P832.
- Trajenta® (linagliptin) Summary of Product Characteristics. SmPCs available at EMC.
- Rosenstock J, et al. Cardiovasc Diabetol. 2018;17:39.
- Rosenstock J, et al. JAMA 2019; 321(1):69-79.
Trajenta is indicated in adults with type 2 diabetes mellitus as an adjunct to diet and exercise to improve glycaemic control as:
monotherapy when metformin is inappropriate due to intolerance, or contraindicated due to renal impairment
in combination with other medicinal products for the treatment of diabetes, including insulin, when these do not provide adequate glycaemic control
PC-GB-110497 | December 2024