Dose & Administration
The simplicity of one dose, once daily to help lower HbA1c in your adult T2D patients1
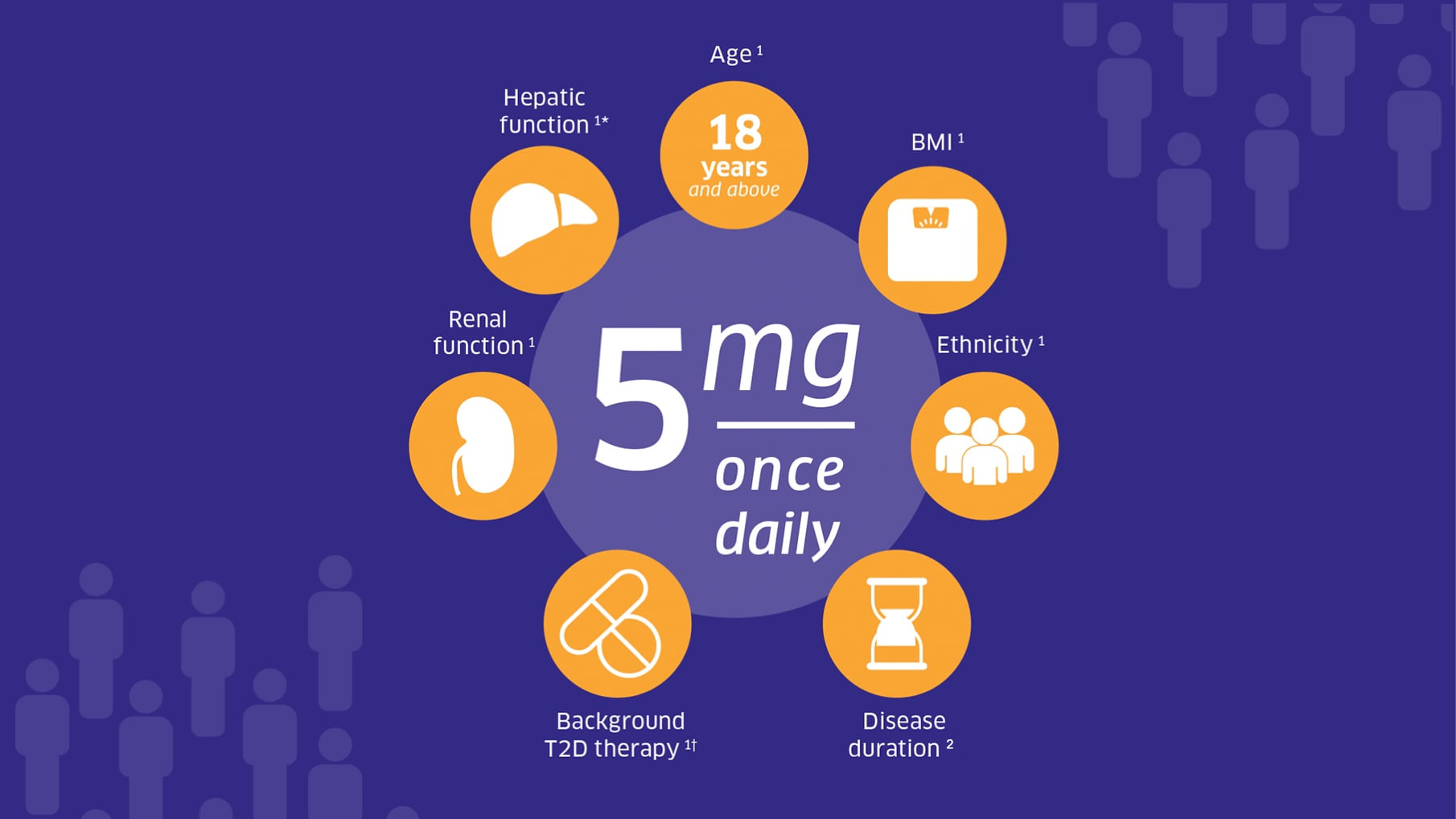
Trajenta® can be used in a broad range of adult patients with a single recommended dose of 5mg, once daily, independent of:

If a dose is missed, it should be taken as soon as the patient remembers. A double dose should not be taken on the same day.1
*Dosing with hepatic impairment: Pharmacokinetic studies suggest that no dose adjustment is required for patients with hepatic impairment but clinical experience in such patients is lacking.1
Dosing in the elderly: No dose adjustment is necessary based on age.1
†Dosing with metformin: When Trajenta® is added to metformin, the dose of metformin should be maintained, and linagliptin administered concomitantly.1
†Dosing with a sulphonylurea or with insulin: Sulphonylureas and insulin are known to cause hypoglycaemia. When Trajenta® is used in combination with a sulphonylurea or with insulin, a lower dose of sulphonylurea or insulin may be considered to reduce the risk of hypoglycaemia.1
Dosing with renal impairment: It is important to ensure that patients are treated according to the licensed indications of a drug, including dose recommendations and monitored in line with clinical guidance including the NICE guidelines on the management of type 2 diabetes and chronic kidney disease (NG28 and NG203).1,3-8
For individual guidance on DPP-4 inhibitor dosing in renal impairment please refer to the individual product SmPC.
| Dosing adjustment based on renal function as defined by SmPC | |||||
|---|---|---|---|---|---|
| Normal function | Mild impairment | Moderate impairment | Severe impairment/ ESRD | ||
| Trajenta® Linagliptin1 | 5 mg OD | ||||
| sitagliptin3 | 100 mg OD | 100 mg OD GFR ≥60 to <90 ml/min | 100 mg OD GFR ≥45 to <60 ml/min | 50 mg OD GFR ≥30 to <45 ml/min | 25 mg OD GFR ≥15 to <30 ml/min; ESRD (GFR <15 ml/ min) including on haemodialysis or peritoneal dialysis |
| vildagliptin4 | 50 mg BD‡‡ 50 mg OD with an SU | 50 mg BD‡‡ 50 mg OD with an SU CrCl 50 to <80 ml/min | 50 mg OD CrCl 30 to <50 ml/min | 50 mg OD CrCl <30 ml/min; ESRD on haemodialysis: use with caution* | |
| saxagliptin5 | 5 mg OD | 5 mg OD | 5 mg OD GFR ≥45 ml/min | 2.5 mg OD** GFR <45 ml/min | 2.5 mg OD** in severe renal impairment; ESRD on haemodialysis: not recommended |
| alogliptin6**†† | 25 mg OD | 25 mg OD CrCl >50 to ≤80 ml/min | 12.5 mg OD CrCl ≥30 to ≤50 ml/min | 6.25 mg OD in severe renal impairment (CrCl <30ml/min); ESRD. Limited experience in renal dialysis. Not studied in peritoneal dialysis | |
Adapted from: 1. Trajenta® SmPC 3. Sitagliptin SmPC 4. Vildagliptin SmPC 5. Saxagliptin SmPC 6. Alogliptin SmPC. Summary of Product Characteristics available at EMC.
GFR: Glomerular filtration rate CrCl: Creatinine Clearance (based on Cockcroft-Gault formula) OD: Once daily BD: Twice daily SU: Sulphonylurea ESRD: End-stage renal disease.
Abbreviations:
BMI: body mass index; DPP-4: dipeptidyl peptidase-4; SmPC: Summary of Product Characteristics; T2D: type 2 diabetes.
Footnotes
-
*
Limited experience in this cohort.4
-
* *
Assessment of renal function is recommended prior to initiation and periodically thereafter.
-
††
Not indicated as monotherapy.
-
‡‡
50mg in the morning and 50mg in the evening.
References
- Trajenta® (linagliptin) Summary of Product Characteristics. SmPCs available at EMC.
- Lajara R, et al. Clin Ther. 2014;36(11):1595-605.
- Sitagliptin Summary of Product Characteristics. SmPCs available at EMC.
- Vildagliptin Summary of Product Characteristics. SmPC available at EMC.
- Saxagliptin Summary of Product Characteristics. SmPCs available at EMC.
- Alogliptin Summary of Product Characteristics. SmPCs available at EMC.
- NICE guidance NG203 November 2021. Available at: https://www.nice.org.uk/guidance/NG203 (last accessed April 2025).
- NICE guidance NG28 February 2022. Available at: https://www.nice.org.uk/guidance/NG28 (last accessed April 2025).
Trajenta is indicated in adults with type 2 diabetes mellitus as an adjunct to diet and exercise to improve glycaemic control as:
monotherapy when metformin is inappropriate due to intolerance, or contraindicated due to renal impairment
in combination with other medicinal products for the treatment of diabetes, including insulin, when these do not provide adequate glycaemic control
PC-GB-110500 V3 | April 2025
