In the UK, current rates of CKD screening, coding and guideline-directed prescribing are low in primary care; earlier identification and treatment could save thousands of lives*4
* Modelling by Kidney Research UK suggestions that improved screening/diagnosis, management, use of SGLT2is and transplant rates could save >10,000 lives between 2023 and 2033.4
Elevated UACR or reduced eGFR is independently associated with a higher risk of CKD progression and CV death5,6
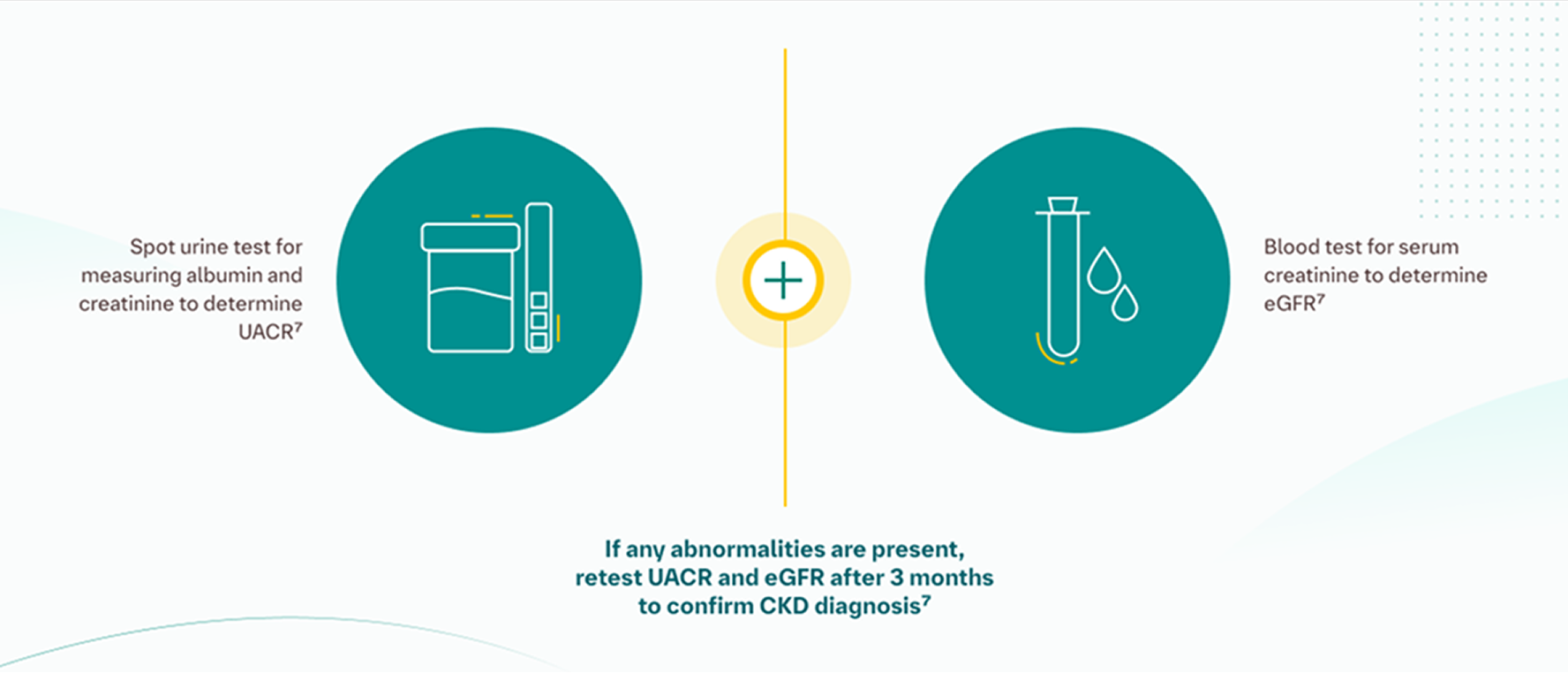
KDIGO and ADA guidelines recommend 2 tests (UACR and eGFR) annually in at-risk individuals as early as possible to adequately assess for kidney damage.7
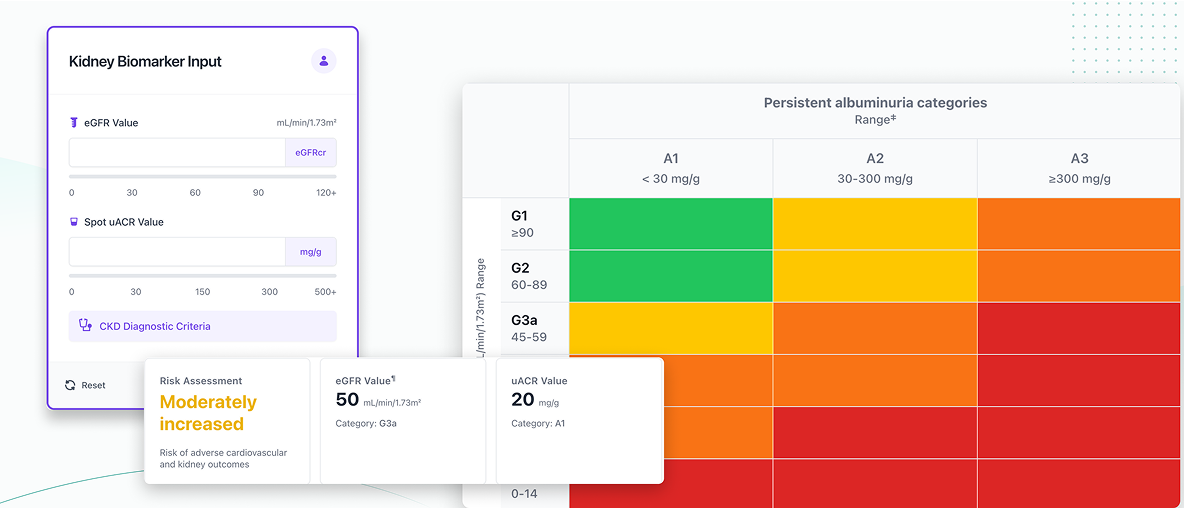
The KDIGO heat map is a useful tool to provide guidance on monitoring and treatment in people with CKD
Access the KDIGO digital heatmap tool to understand the cardiovascular and renal risk associated with the CKD stage of your patient.
The above tool was developed by Boehringer Ingelheim in collaboration with KDIGO.
-
*
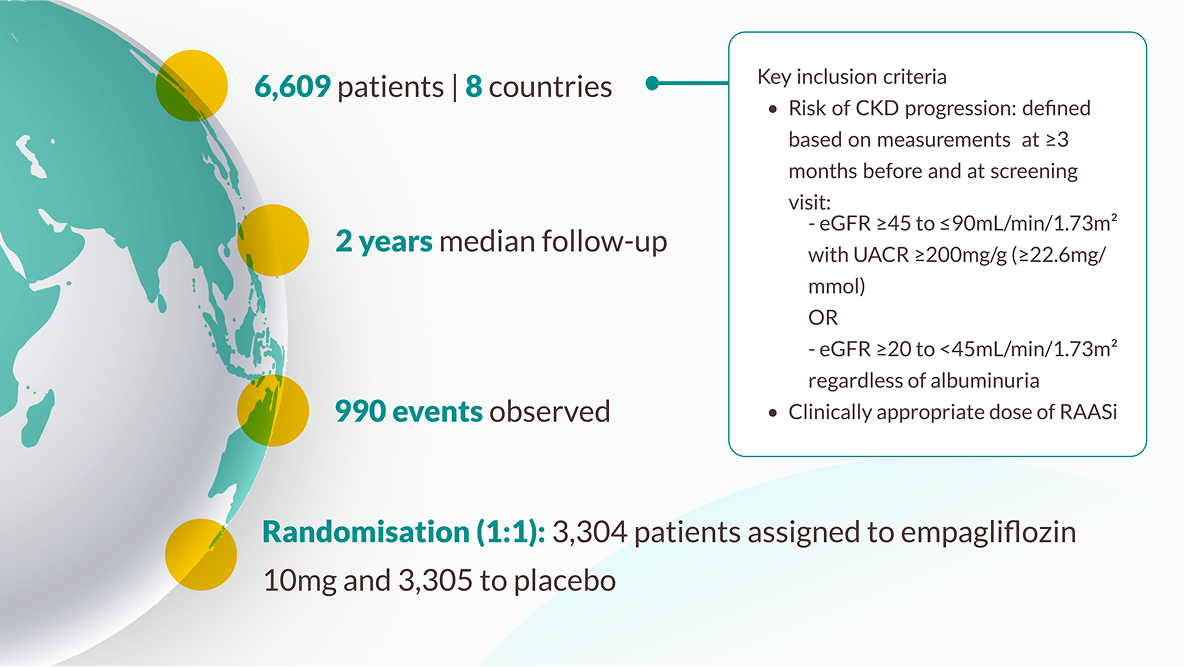
The trial enrolled 6,609 patients who had evidence of CKD at risk of kidney disease progression, with and without diabetes, with and without albuminuria. Patients with an eGFR ≥20 to <45 mL/min/1.73m2, or an eGFR ≥45 to <90 mL/ min/1.73m2 with a UACR ≥200mg/g, were randomised to receive either 10mg empagliflozin (n=3304) or placebo (n=3305) on top of standard of care. JARDIANCE® achieved primary endpoint vs placebo: risk reduction in kidney disease progression or CV death 28% RRR/3.6% ARR (HR=0.72; 95% CI: 0.64, 0.82; p<0.001). Kidney disease progression was defined as end-stage kidney disease (ESKD: the initiation of maintenance dialysis or receipt of a kidney transplant), a sustained decrease in the eGFR to <10 mL/min/1.73m2 , a sustained decrease from baseline in the eGFR of at least 40%, or death from renal causes.9,10
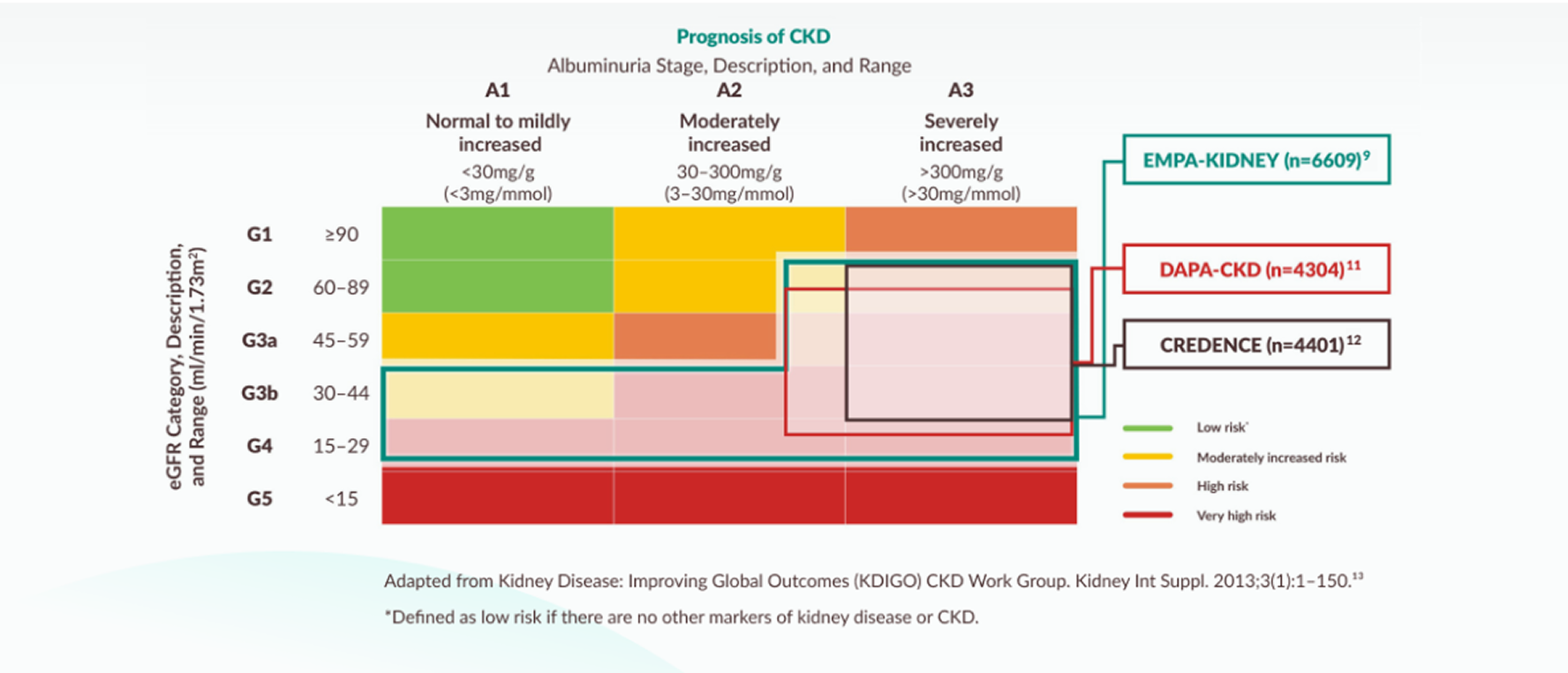
EMPA-KIDNEY included the broadest range of patients amongst the SGLT2i CKD RCTs in terms of eGFR and those with and without albuminuria9,11,12
Inclusion criteria of the SGLT2i CKD trials mapped to KDIGO guidance
NICE 2023 TA942 recommendation for the use of empagliflozin in adults with CKD when mapped to the KDIGO 2024 guidance on CKD prognosis by eGFR and albuminuria categories. It is recommended as an add-on to optimised standard care including the highest tolerated licensed dose of ACE inhibitors or ARBs, unless these are contraindicated.14
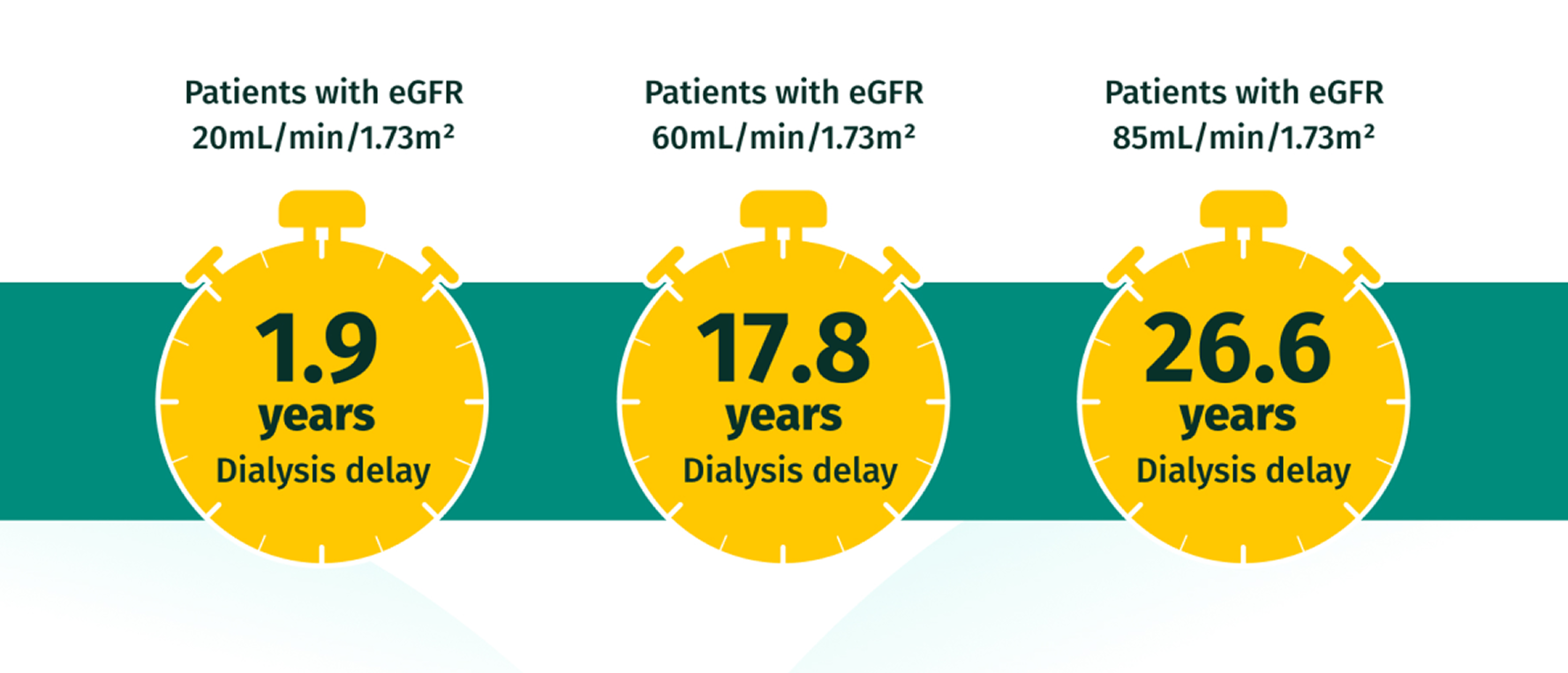
EARLY intervention with JARDIANCE® may help patients avoid dialysis for longer15
Potential impact on time to dialysis versus SoC based on extrapolated data from the EMPA-KIDNEY trial
Based on hypothetical transformation of chronic eGFR slopes into time to kidney failure, i.e., time to need for kidney replacement therapy, defined as eGFR = 10mL/min/1.73m2. Data estimated from each baseline eGFR value by applying the chronic eGFR slopes corresponding to participants on standard of care vs empagliflozin within the prespecified eGFR subgroups (eGFR cutoff points to define subgroups).
Latest resource

Webinar - 5 minute highlight - BEYOND THE FILTER
Watch Professor Smeeta Sinha and Dr Sarah Jarvis explore the role of SGLT2 inhibitors in CKD management—as they discuss the burden of CKD, current treatment approaches including uACR testing, key insights from the EMPA-KIDNEY trial, and prescribing considerations for empagliflozin.
Prefer to watch the full recording? Click here to view the full webinar recording.
JARDIANCE® patient booklets

JARDIANCE® Initiation & Management guide for T2D, CHF and CKD

JARDIANCE® patient booklet
Abbreviations
ADA: American Diabetes Association; ARR: absolute risk reduction; CI: confidence interval; CRM: cardio renal metabolism; CV: cardiovascular; CVD: cardiovascular disease; HR: hazard ratio; KDIGO: Kidney Disease: Improving Global Outcomes; MACE: major adverse cardiovascular events; MI: myocardial infarction; NICE: National Institute for Health and Care Excellence; PAD: peripheral artery disease; QOF: quality and outcomes framework; RCT: randomised controlled trial; RRR: relative risk reduction; SGLT2: sodium-glucose co-transporter-2; SGLT2i: sodium-glucose co-transporter-2 inhibitor; SoC: standard of care; UKKA: UK Kidney Association.
- GBD Chronic Kidney Disease Collaboration. Lancet. 2020;395(10225):709–733.
- Jankowski J, et al. Circulation. 2021;143(11):1157–1172.
- Borg R, et al. Int J Nephrol. 2023 Mar
- Kidney Research UK. Kidney disease: a UK public health emergency. The health economics of kidney disease to 2033. 2023. Available at: https://www.kidneyresearchuk.org/wp-content/uploads/2023/06/Economics-of-Kidney-Disease-full-report_accessible.pdf.
- Choi Y et al. J Am Heart Assoc. 2022;11:e026685.
- van der velde M et al. Kidney International. (2011);79:1341–1352.
- de Boer IH, et al. Diabetes Care. 2022;45(12):3075–3090.
- JARDIANCE (empagliflozin) Summary of Product Characteristics (SmPC).
- Herrington WG, et al. N Engl J Med. 2023;388(2):117-127. (EMPA-KIDNEY results and the publication’s Supplementary Appendix).
- JARDIANCE Data on File (EMP 23-22).
- Heerspink HJL et al. N Engl J Med. 2020;383:1436–1446.
- Perkovic V et al. N Engl J Med. 2019;380:2295–2306.
- Kidney Disease: Improving Global outcomes (KDIGO) CKD Work Group. Kidney Int.2024;105(4S):S117-S314.
- NICE. Empagliflozin for treating chronic kidney disease: Technology appraisal guidance (TA942). 2023. Available from: https://www.nice.org.uk/guidance/ta942/.
- Fernández-Fernandez B, Sarafidis P, Soler MJ, Ortiz A. Clin Kidney J. 2023;16(8):1187–1198.
PC-GB-111790 | August 2025
The content on this website is in relation to adult patients.
Empagliflozin is not recommended in severe hepatic impairment, breastfeeding, Type 1 diabetes and is contraindicated in patients with hypersensitivity to the active ingredient or any of its excipients. Empagliflozin should be avoided in pregnancy.
Please consult the SmPC for full details regarding adverse events, monitoring requirements and interactions prior to prescribing JARDIANCE®.
- JARDIANCE® (empagliflozin) UK Summary of Product Characteristics (SmPC). Available at:
http://www.medicines.org.uk/emc/medicine/28973.
JARDIANCE® is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise
- as monotherapy when metformin is considered inappropriate due to intolerance
- in addition to other medicinal products for the treatment of diabetes1
JARDIANCE® is indicated in adults for the treatment of chronic kidney disease.1
JARDIANCE® is indicated in adults for the treatment of symptomatic chronic heart failure.1
PC-GB-109995 V4 | October 2025