
EMPA-REG OUTCOME® trial

EMPA-REG MET® trial
EMPA-REG OUTCOME® trial
Composite primary endpoint:
- 3P-MACE (comprising cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke)
Key secondary endpoint:
- 4P-MACE (comprising cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, and hospitalisation for unstable angina)
Exploratory endpoints:
- Cardiovascular death
- All-cause mortality
- Hospitalisation for heart failure
-
*
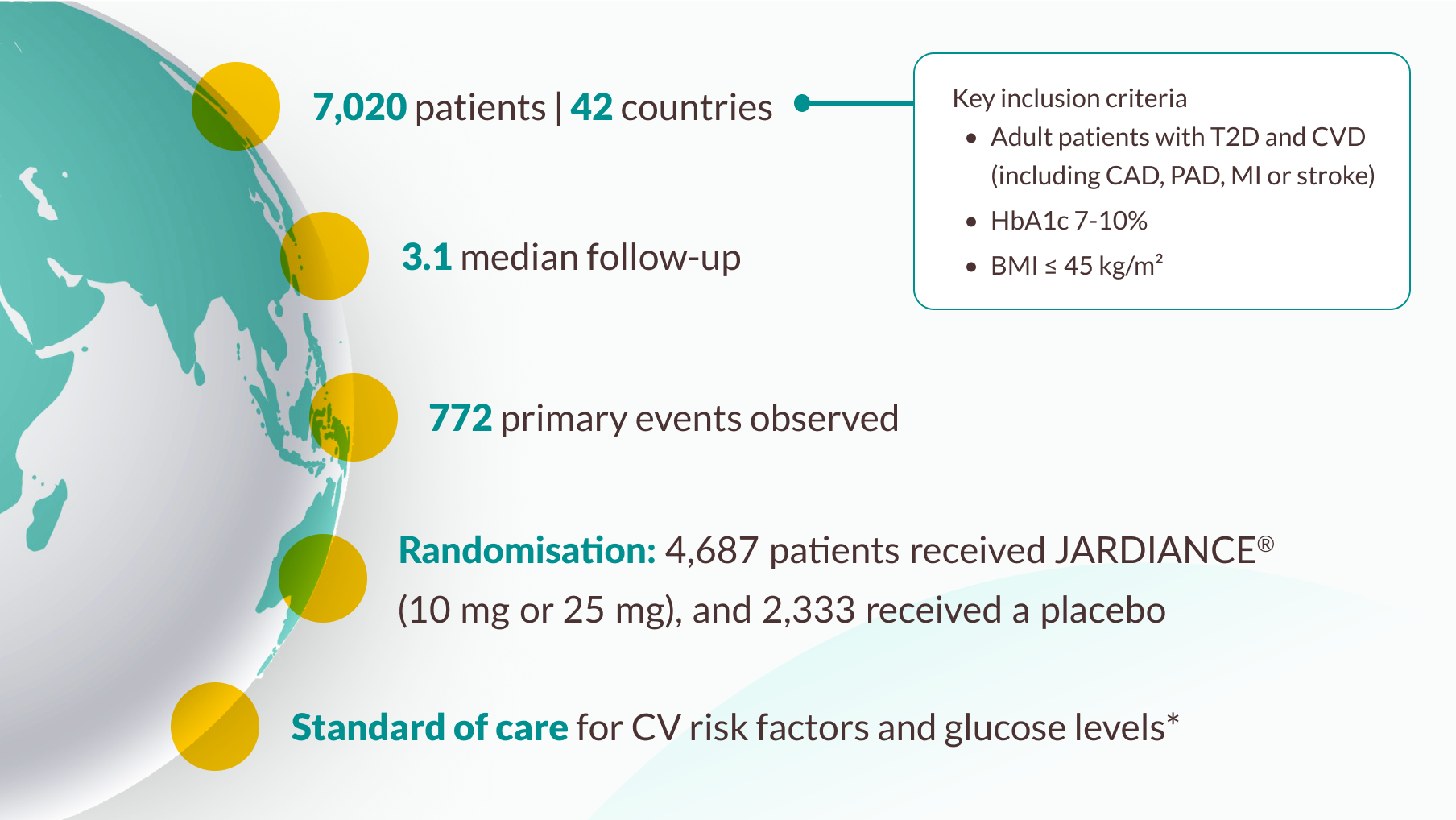
Investigators managed cardiovascular risk factors according to standard care. Background glucose-lowering therapy remained unchanged for the first 12 weeks, with adjustments allowed only for confirmed high fasting glucose (>13.3 mmol/L). After 12 weeks, glucose-lowering therapy could be adjusted according to local guidelines for glycaemic control.
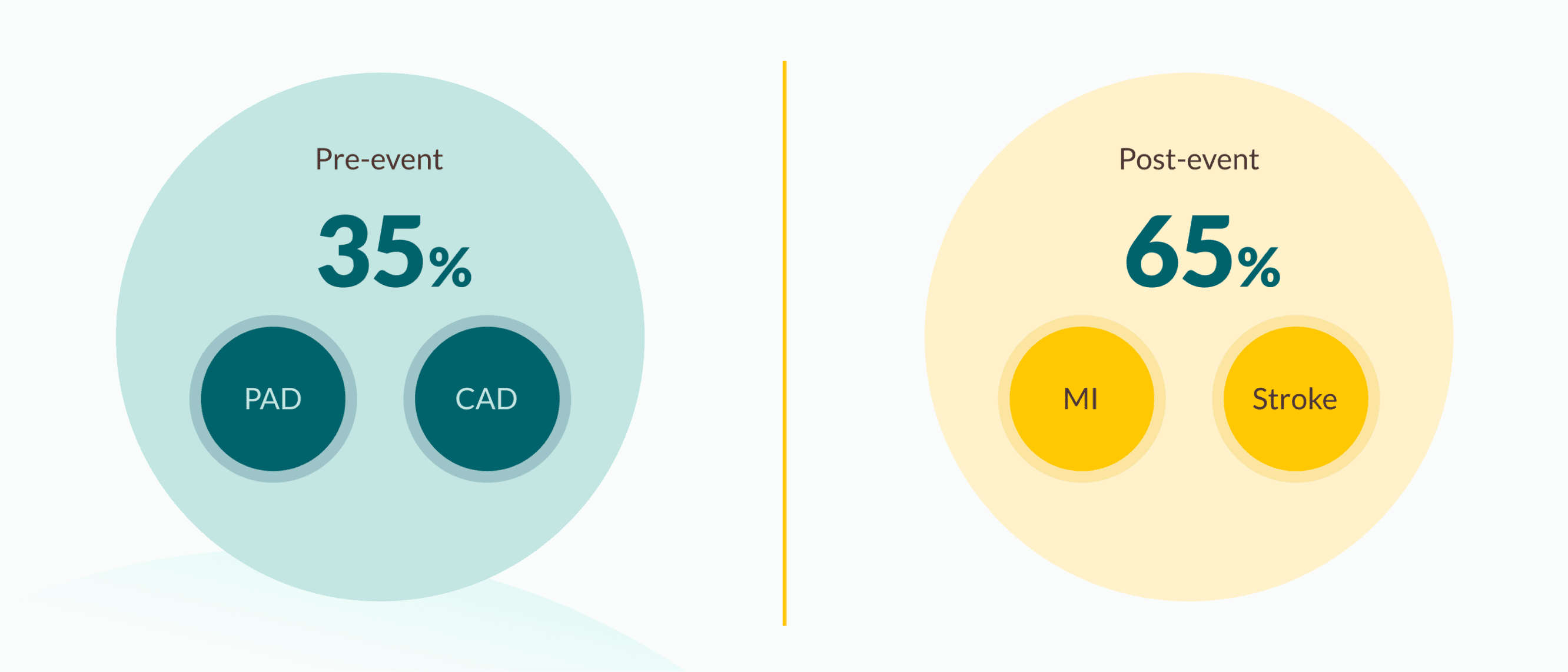
1/3 of patients in the EMPA-REG OUTCOME® trial had not experienced a previous myocardial infarction (MI) and/or stroke
The EMPA-REG OUTCOME® trial studied over 7,000 patients with T2D and CVD1
EMPA-REG OUTCOME® population2
(split was the same across both pooled empagliflozin and placebo groups)
Patient characteristics
Background medication for patients who received JARDIANCE®1,3
Antidiabetic drugs | Antihypertensives |
Statins | Anticoagulants* |
-
*
For the purposes of this study, "anticoagulants" refers to both anticoagulants and antiplatelets, as outlined in the study protocols.
Patients who were treated with JARDIANCE®1
At baseline, demographic and clinical characteristics were well balanced between the placebo group and the empagliflozin group.
63 yearsaverage age | 76%coronary artery disease | 47%history of myocardial infarction |
23%history of stroke | 
26%eGFR <60 ml/min/1.73m2 | 
21%peripheral artery disease |
Tolerability profile1
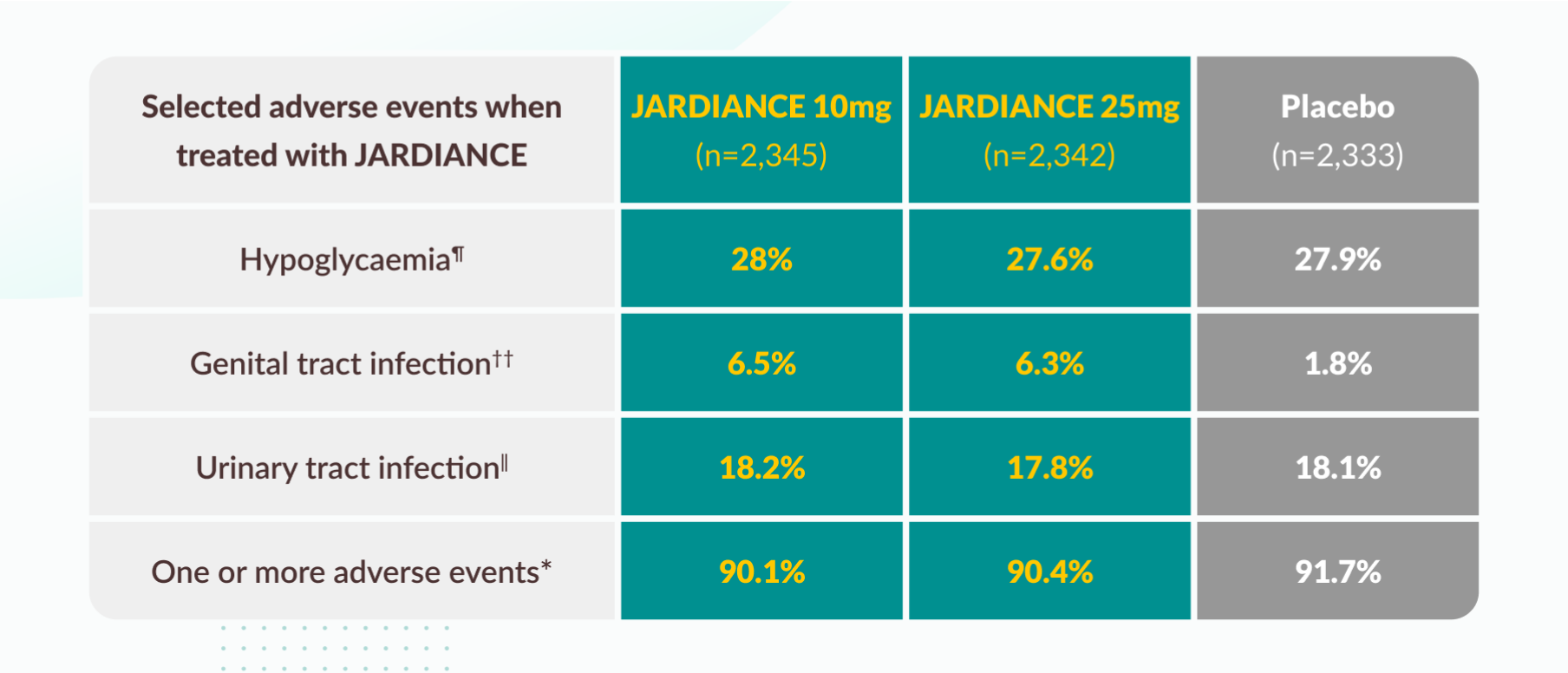
-
¶
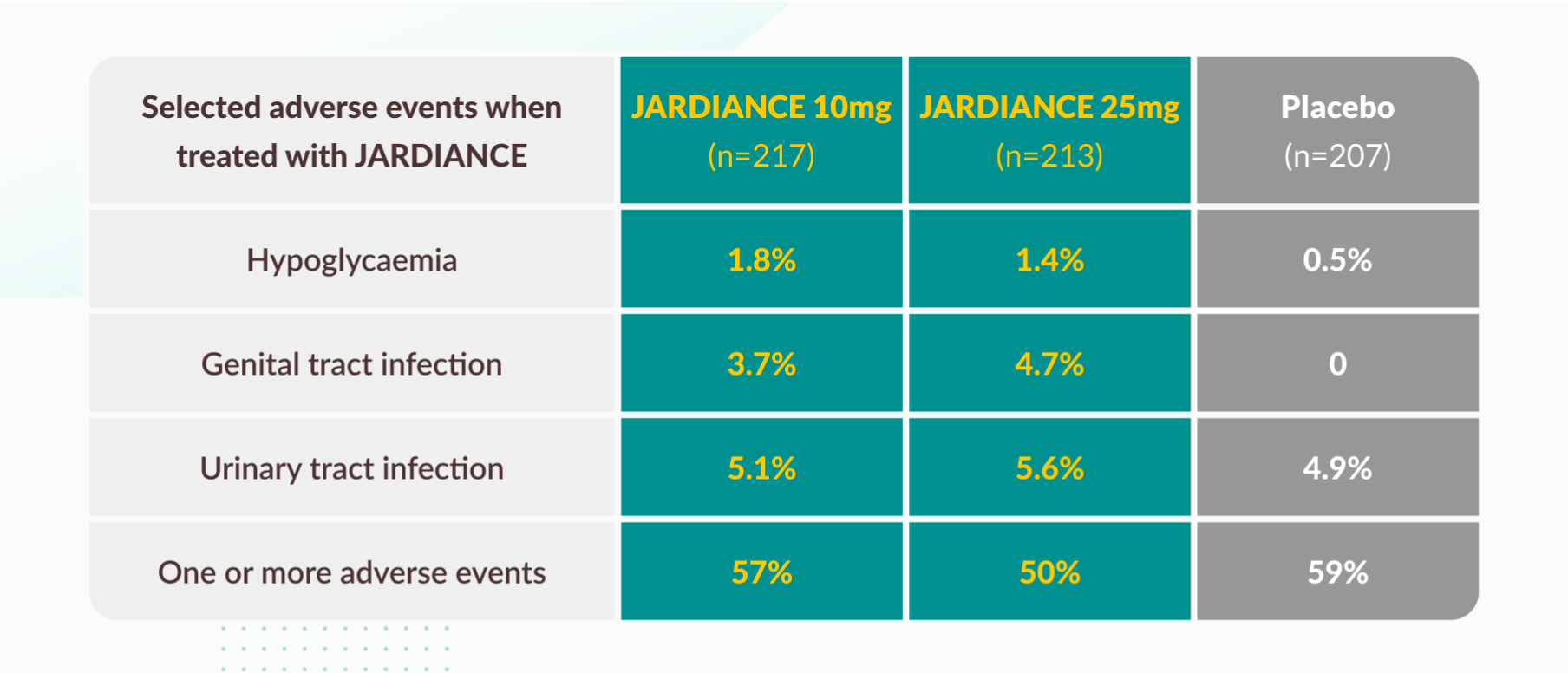
A confirmed hypoglycemic adverse event was a plasma glucose level of less than 70 mg per deciliter (3.9 mmol per liter) or an event requiring assistance.
-
‖
The definition of urinary tract infection was based on 79 preferred terms in the Medical Dictionary for Regulatory Activities (MedDRA). Percentages were calculated as the proportions of all men and all women with the event.
-
††
The definition of genital infection was based on 88 MedDRA preferred terms. Percentages were calculated as the proportions of all men and all women with the event.
-
*
Data are for patients who had one or more events and who had received at least one dose of a study drug. All events occurred within 7 days after the last receipt of the study drug.
Urinary tract infections
No significant increase compared with placebo4
Genital infections
Occurred only once in the majority of patients5
Mostly mild to moderate in intensity6
Generally treatable with standard therapies in most cases7
Increased urination
Mostly mild to moderate intensity:6
JARDIANCE® 10 mg and 25 mg: 3.5% and 3.3% compared with placebo: 1.4%6
Frequency of nocturia was similar with placebo (<1%)6
Patients with type 2 diabetes are generally more susceptible to urogenital tract infections8
Efficacy results: EMPA-REG OUTCOME trial
Primary outcome of EMPA-REG OUTCOME
The primary outcome of 3P-MACE occurred in a significantly lower percentage of patients in the empagliflozin group (490 of 4687 [10.5%]) than in the placebo group (282 of 2333 [12.1%]) (hazard ratio in the empagliflozin group, 0.86; 95.02% confidence interval [CI], 0.74 to 0.99; P<0.001 for noninferiority and P=0.04 for superiority).1
Key secondary outcome of EMPA-REG OUTCOME
The key secondary outcome, 4P MACE, occurred in 599 of 4687 patients (12.8%) in the empagliflozin group and 333 of 2333 patients (14.3%) in the placebo group (hazard ratio, 0.89; 95% CI, 0.78 to 1.01; P<0.001 for noninferiority and P=0.08 for superiority).1
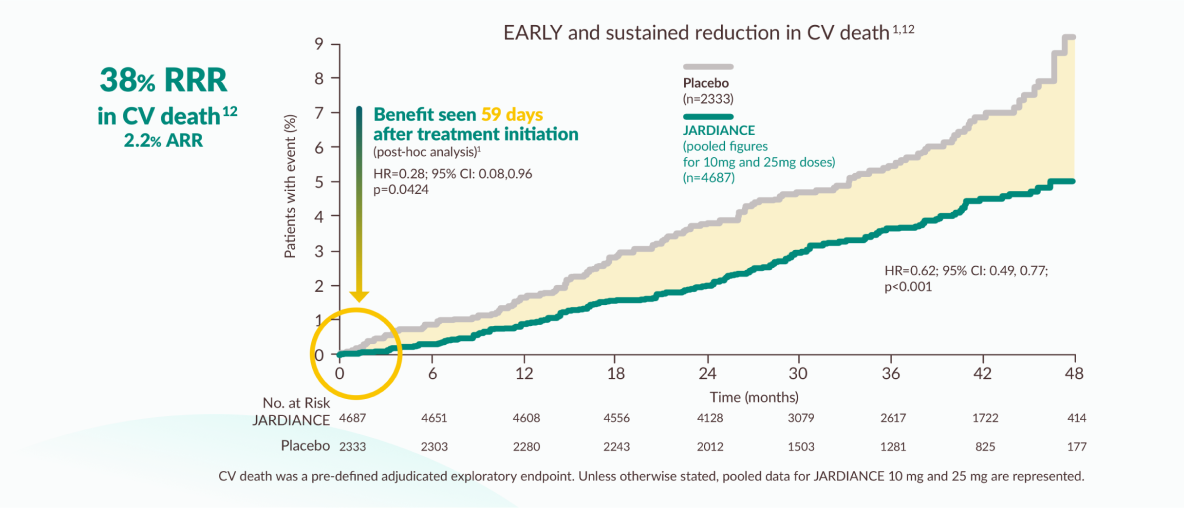
Pre-defined exploratory outcome and post-hoc analysis of EMPA-REG OUTCOME
JARDIANCE® reduced the risk of CV death as EARLY as 59 days9
The efficacy for preventing cardiovascular mortality has not been conclusively established in patients using JARDIANCE® concomitantly with DPP-4 inhibitors or in black patients because the representation of these groups in the EMPA-REG OUTCOME trial was limited.6
Improvements were seen:
- Independent of HbA1c, BMI, or blood pressure6,9,10
- On top of standard of care for T2D and CV disease (statins, ACE inhibitors/ ARBs, beta blockers, antiplatelets/anticoagulants, glucose-lowering medications)1
Pre-defined exploratory outcome—All-cause mortality of EMPA-REG OUTCOME
JARDIANCE® can help reduce the risk of death from any cause, with 1 life saved for every 39 patients treated over 3 years1
32% RRR, 2.6% ARR
269 events (5.7%) in the empagliflozin group vs. 194 events (8.3%) in the placebo group; HR 0.68, 95% CI 0.57–0.82, p<0.001.
These numbers cannot be extrapolated to patient populations with other clinical characteristics.
Want to discuss further?

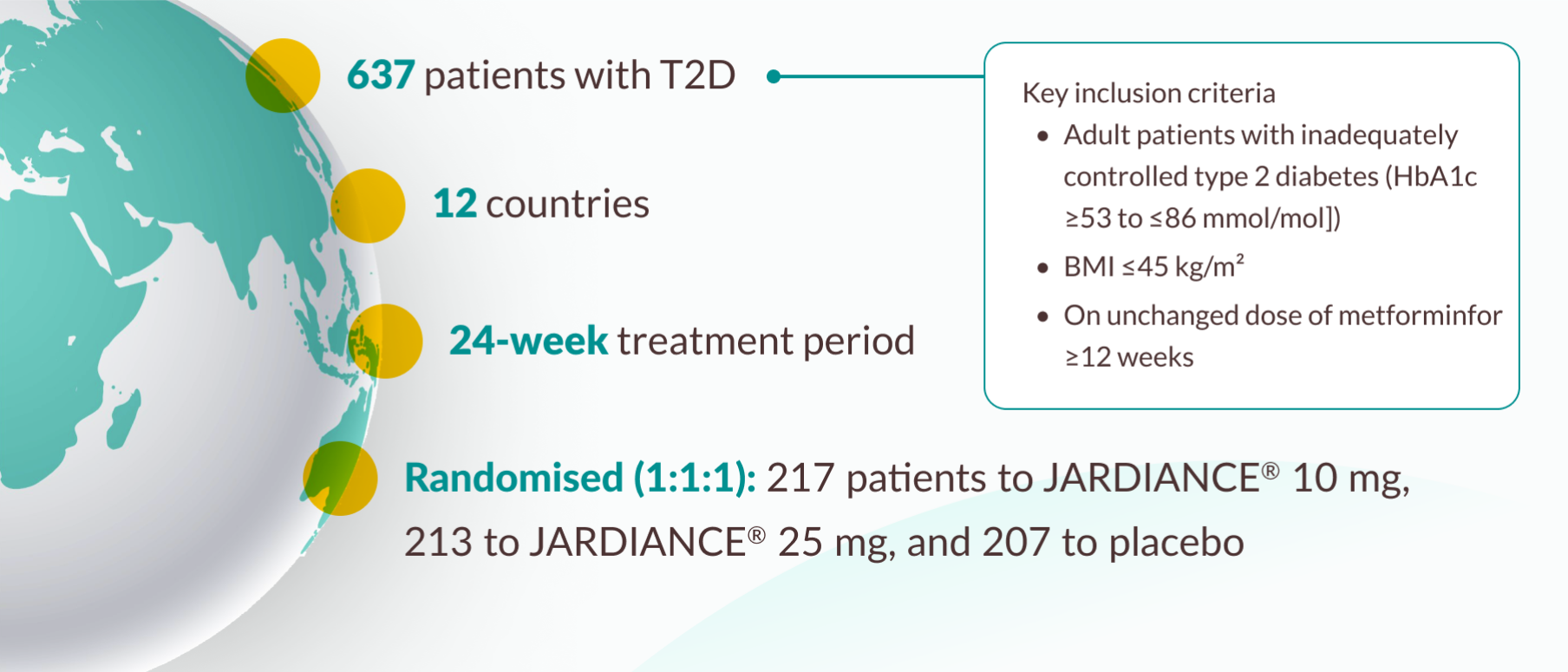
EMPA-REG MET® trial
Primary endpoint:
Change from baseline in HbA1c level at week 24
Key secondary endpoints:
Change from baseline to week 24 in body weight
Change in weighted mean daily glucose (MDG) using an 8-point blood glucose profile
Exploratory endpoints:
Change from baseline to week 24 in fasting plasma glucose (FPG), systolic blood pressure (SBP), and diastolic blood pressure (DBP)
Proportion of patients achieving HbA1c <7% (<53 mmol/mol) at week 24
Proportion of patients with >5% reduction in body weight at week 24
Proportion of patients with controlled blood pressure (SBP <130 mmHg and DBP <80 mmHg) at week 24
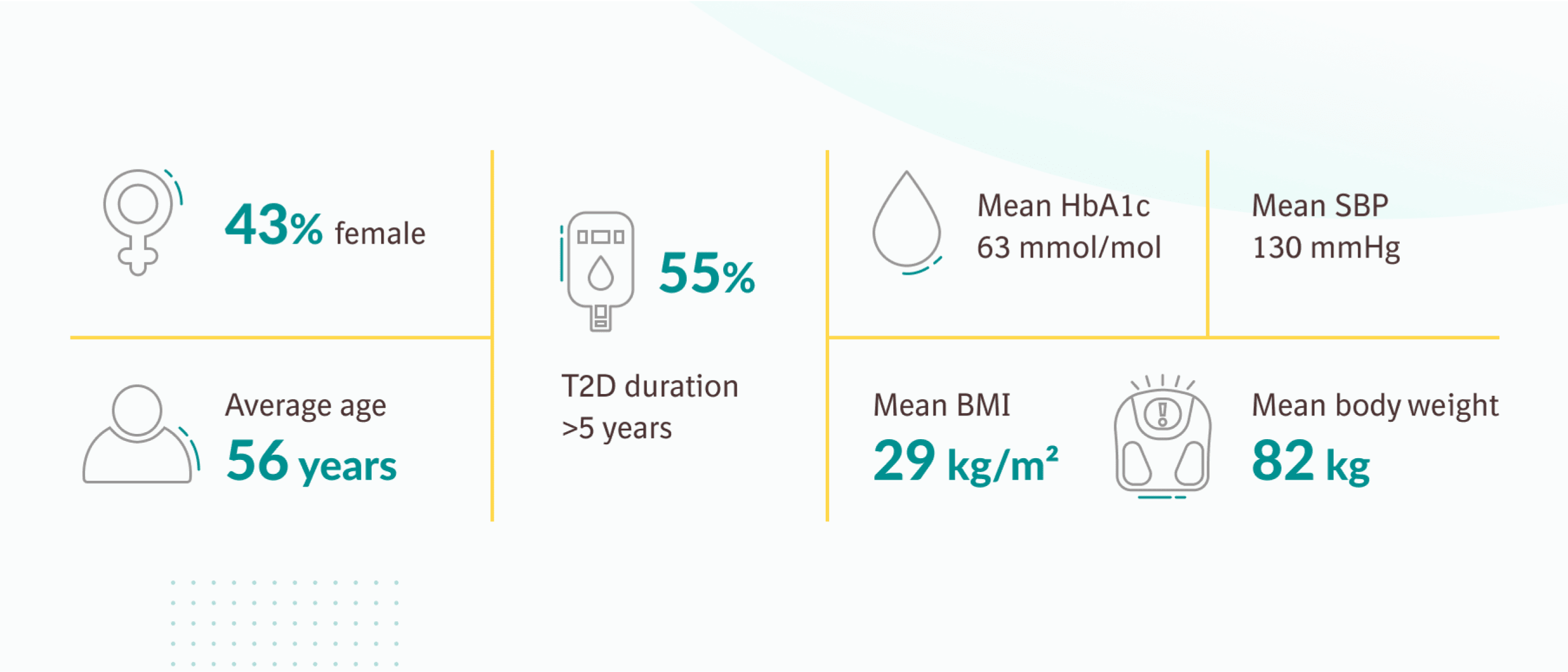
Patient characteristics11
Patient characteristics represent the entire study cohort from the EMPA-REG MET® trial.11
White | Asian | Black/African American |
Baseline characteristics of patients who received JARDIANCE®11
Tolerability profile11
The number of patients reporting one or more AEs was similar across groups. Most patients with one or more AEs (95%) reported only events of mild or moderate intensity.
Efficacy results: EMPA-REG MET trial
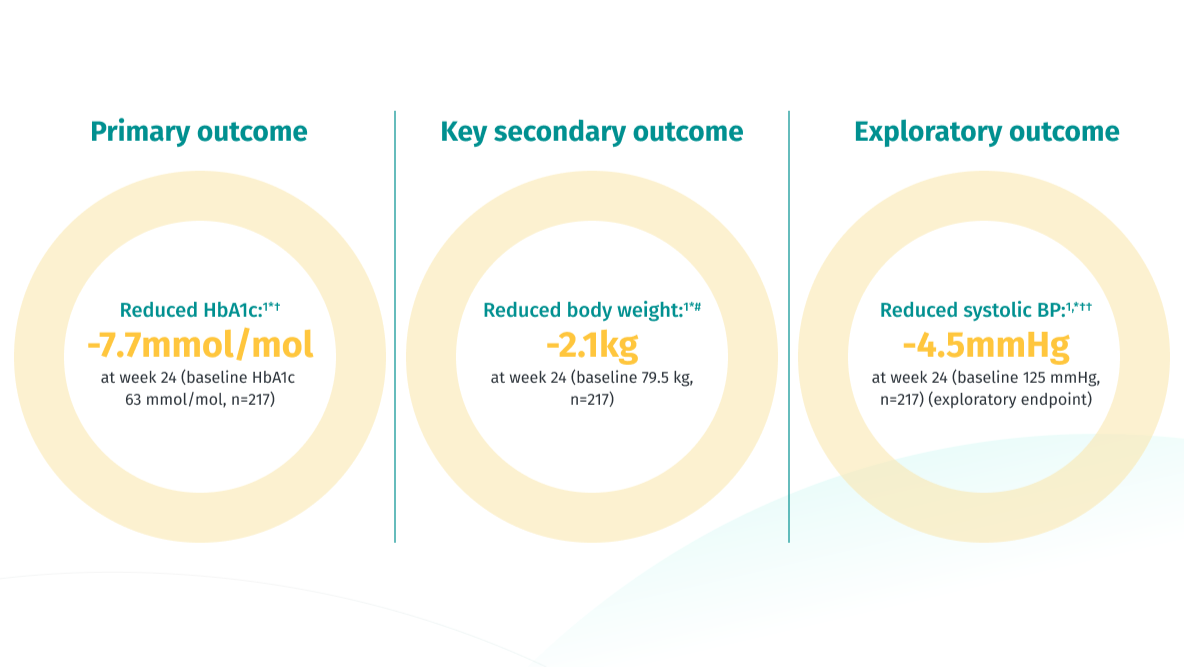
For your T2D patients, accomplish more than just HbA1c reduction11
In the EMPA-REG MET trial JARDIANCE® 10mg demonstrated superior outcomes vs placebo in reducing HbA1c, body weight, and systolic blood pressure (exploratory endpoint) (p<0.001).
90% of weight loss with JARDIANCE® is due to reduction in fat mass11
Please note that all data shown is for JARDIANCE® 10mg. JARDIANCE® is not licensed for weight or blood pressure reduction.
Want to discuss further?
-
*
In a 24-week, double-blind, placebo-controlled study of 637 patients with type 2 diabetes, the efficacy and safety of JARDIANCE® 10mg (n=217) and JARDIANCE® 25mg (n=213) as add-on therapy to metformin ≥1500mg were evaluated vs placebo added to metformin (n=207). The primary endpoint was adjusted mean change (SE) from baseline in HbA1c (%) at 24 weeks; change from baseline in weight and blood pressure at week 24 were key secondary and exploratory endpoints, respectively.11
-
†
Adjusted mean changes from baseline HbA1c 7.9% were -0.13% for placebo (n=207), -0.7% for JARDIANCE 10mg (n=217) and -0.77% for JARDIANCE® 25mg (n=213), respectively. Difference from baseline vs placebo (adjusted mean) was -0.6 % for both JARDIANCE® 10mg and 25mg; p<0.001 vs placebo for both doses.11
-
#
Adjusted mean changes of -0.5kg reduction in body weight from baseline 79.7kg for placebo (n=207), -2.1kg from baseline 81.6kg for JARDIANCE® 10mg (n=217), and -2.5kg from baseline 82.2kg for JARDIANCE® 25mg (n=213), p<0.001 vs placebo for both doses.11
-
††
Change in systolic blood pressure from baseline at 24 weeks: JARDIANCE® 10mg -4.5mmHg (n=217), JARDIANCE® 25mg -5.2mmHg (n=213), vs placebo -0.4mmHg (n=207). Mean baseline value for JARDIANCE® 10mg 129.6mmHg, 25mg 130.0mmHg and placebo 128.6mmHg (p<0.001 vs placebo for both doses).11
Knowledge base
JARDIANCE® is simple to initiate at 10 mg dosing across all indications.

Adverse reactions from reported placebo-controlled studies and post-marketing experience.

Abbreviations
ARR: absolute risk reduction; BMI: body mass index; BP: blood pressure; CAD: coronary artery disease; CVD: cardiovascular disease; eGFR: estimated glomerular filtration rate; HbA1c: haemoglobin A1c; MI: myocardial infarction; PAD: peripheral arterial disease; RRR: relative risk reduction; T2D: type 2 diabetes.
- Zinman B, et al. N Engl J Med. 2015;373:2117–2128.
- Fitchett D, et al. Circulation. 2019;139(11):1384–1395.
- Fitchett D, et al. Poster no. 301 presented at: European Society of Cardiology Heart Failure 2017 and 4th World Congress on Acute Heart Failure; April 29–May 2, 2017; Paris, France.
- Kohler S et al. Adv Ther 2017; 34(7): 1707–1726.
- Kim G et al. American Diabetes Association 73rd Scientific Sessions, 21.–25. Juni 2013, Chicago; Poster 74-LB.
- JARDIANCE® (empagliflozin) Summary of Product Characteristics (SmPC). Available at: http://www.medicines.org.uk/emc/medicine/28973.
- Arakaki RF. J Postgrad Med 2016; 128(4): 409–417.
- Geerlings S et al. Diabetes Res Clin Pract 2014; 103(3): 373–381.
- Verma S, et al. ESC Heart Fail. 2021;8:2603-2607.
- Inzucchi SE, et al. Circulation. 2018;138:1904–1907.
- Haring HU, et al. Diabetes Care. 2014;37:1650–1659.
- Fitchett D, et al. J Am Coll Cardiol. 2018;71:364–367.
PC-GB-110619 | December 2024
The content on this website is in relation to adult patients.
Empagliflozin is not recommended in severe hepatic impairment, breastfeeding, Type 1 diabetes and is contraindicated in patients with hypersensitivity to the active ingredient or any of its excipients. Empagliflozin should be avoided in pregnancy.
Please consult the SmPC for full details regarding adverse events, monitoring requirements and interactions prior to prescribing JARDIANCE®.
- JARDIANCE® (empagliflozin) UK Summary of Product Characteristics (SmPC). Available at:
http://www.medicines.org.uk/emc/medicine/28973.
JARDIANCE® is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise
- as monotherapy when metformin is considered inappropriate due to intolerance
- in addition to other medicinal products for the treatment of diabetes1
JARDIANCE® is indicated in adults for the treatment of chronic kidney disease.1
JARDIANCE® is indicated in adults for the treatment of symptomatic chronic heart failure.1
PC-GB-109995 V4 | October 2025

