Learn more about JARDIANCE® for:

Type 2 diabetes (T2D)
Chronic kidney disease (CKD)
Symptomatic chronic heart failure (CHF)
Abbreviations
CHF: chronic heart failure; CKD: chronic kidney failure; CRM: cardio-renal-metabolic; CV: cardiovascular; eGFR: estimated glomerular filtration rate; HbA1c: glycated haemoglobin; LV: left ventricular; SGLT2i: sodium-glucose co-transporter-2 inhibitor; T2D: type 2 diabetes mellitus.
- JARDIANCE® (empagliflozin) Summary of Product Characteristics (SmPC). Available at: http://www.medicines.org.uk/emc/medicine/28973.
- Torimoto K et al. Diabetol Metab Syndr. 2017;9:60.
- Ferrannini G et al. Diabetes Care. 2015;38:1730–1735.
- Ridderstråle M et al. Lancet Diabetes Endocrinol. 2014;2:691–700.
- Garg V et al. Prog Cardiovasc Dis. 2019;62:349–357.
- Wanner C et al. N Engl J Med. 2016; 375:323–334.
- Häring HU, et al. Diabetes Care. 2014;37:1650–1659.
- Kalra S. Diabetes Ther. 2014 Dec;5:355-66.
PC-GB-110622 | December 2024
The content on this website is in relation to adult patients.
Empagliflozin is not recommended in severe hepatic impairment, breastfeeding, Type 1 diabetes and is contraindicated in patients with hypersensitivity to the active ingredient or any of its excipients. Empagliflozin should be avoided in pregnancy.
Please consult the SmPC for full details regarding adverse events, monitoring requirements and interactions prior to prescribing JARDIANCE®.
- JARDIANCE® (empagliflozin) UK Summary of Product Characteristics (SmPC). Available at:
http://www.medicines.org.uk/emc/medicine/28973.
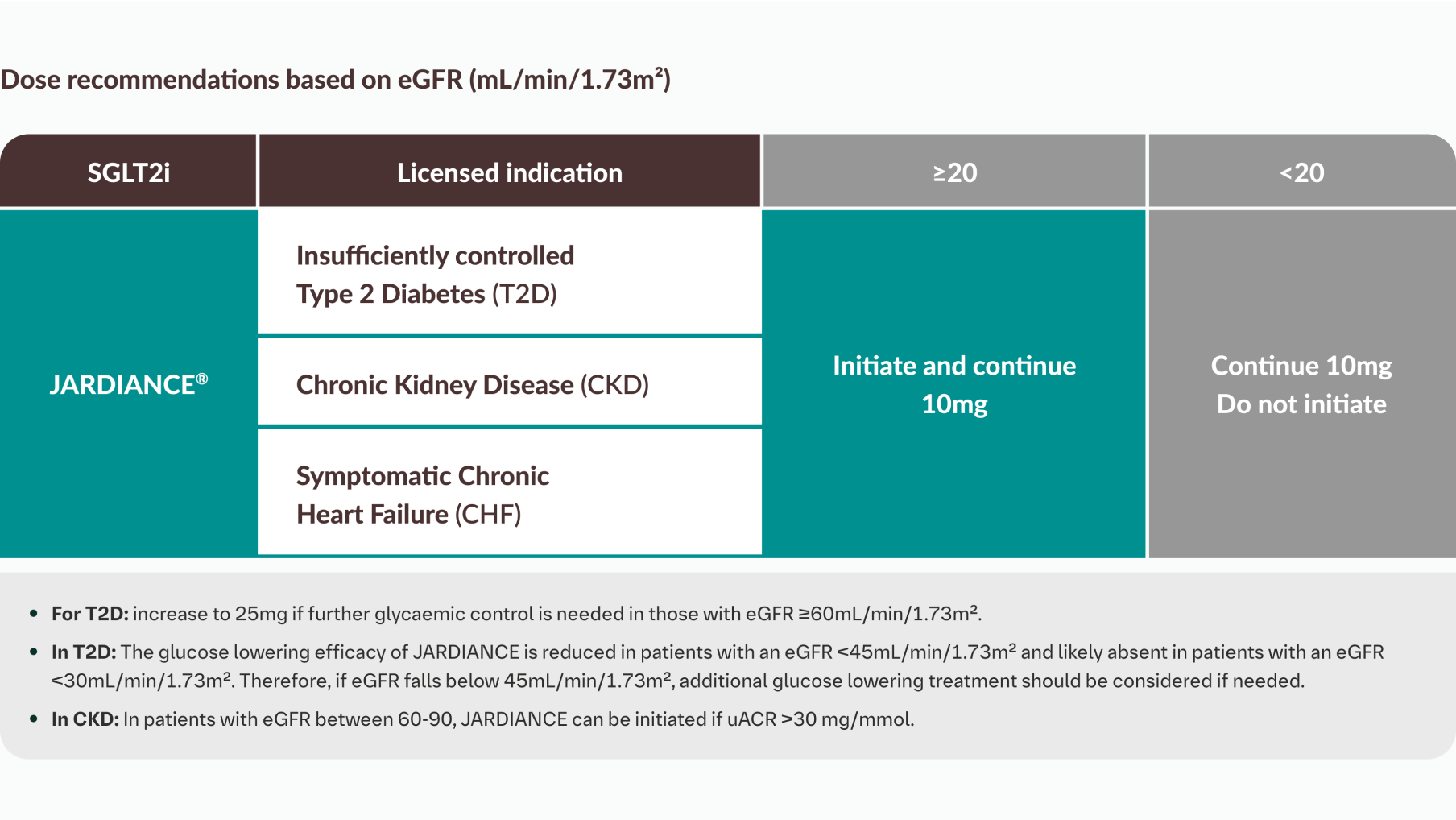
JARDIANCE® is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise
- as monotherapy when metformin is considered inappropriate due to intolerance
- in addition to other medicinal products for the treatment of diabetes1
JARDIANCE® is indicated in adults for the treatment of chronic kidney disease.1
JARDIANCE® is indicated in adults for the treatment of symptomatic chronic heart failure.1
PC-GB-109995 V4 | October 2025