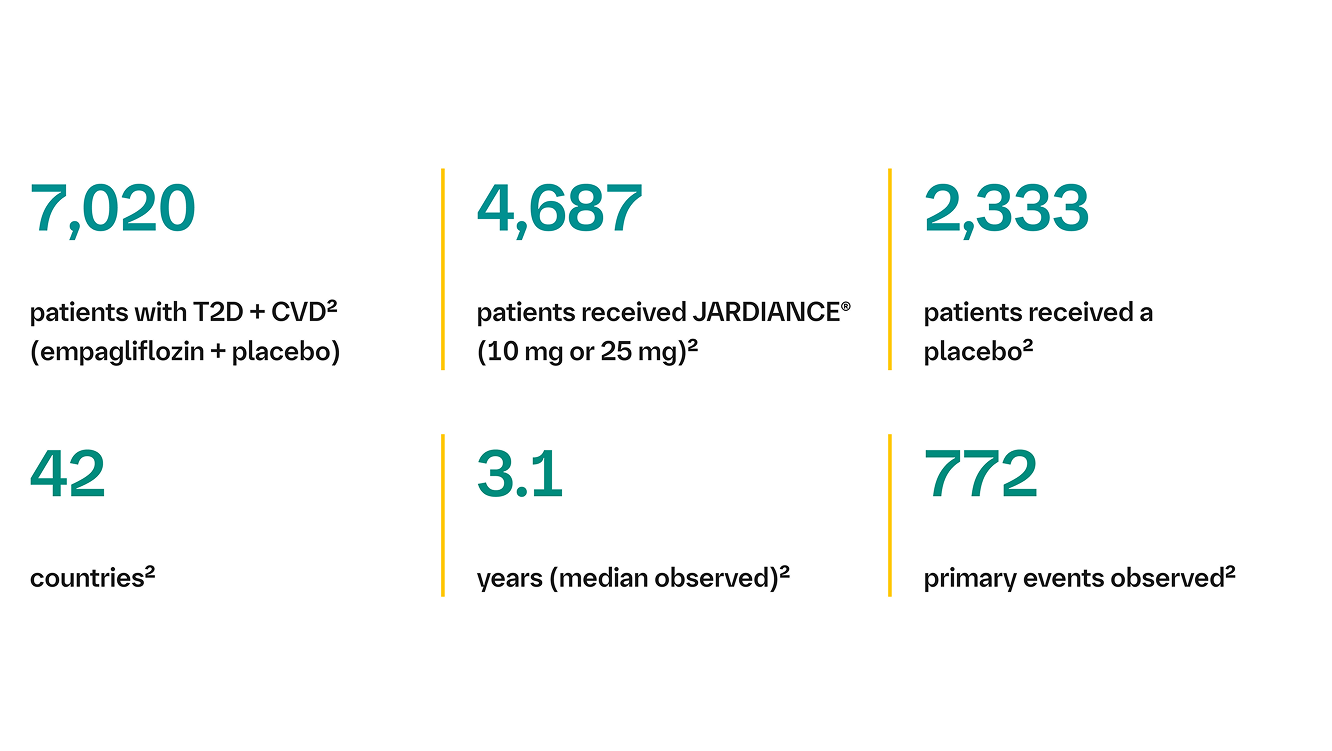
The EMPA-REG OUTCOME® trial, published in the NEJM, demonstrated for the first time that a glucose-lowering agent also reduced the risk of cardiovascular and renal events in patients with T2D and CVD on top of standard of care.2
Standard of care: gluclose-lowering therapies (98%), antihypertensives (95%), antiplatelets/anticoagulants (89%) and statins (77%)a



2025
The lasting impact of the EMPA-REG OUTCOME®:
JARDIANCE® has an established safety and tolerability profile1
JARDIANCE® had a generally consistent safety profile across the studied indications. Very common adverse events (≥1/10) are hypoglycaemia (when used with sulphonylurea or insulin) and volume depletion. Common adverse events (≥1/100 to <1/10) are UTIs, GTIs, thirst, constipation, pruritus, rash, increased urination, and serum lipids increase. JARDIANCE® is not recommended in severe hepatic impairment, should not be used in breastfeeding or type 1 diabetes, and is contraindicated in patients with hypersensitivity to the active ingredient or any of its excipients. JARDIANCE® should be avoided in pregnancy.
For a complete list of AEs, contraindications, warnings, and precautions, please refer to the Summary of Product Characteristics.
Footnotes
-
a.
Standard of care included CV medications and glucose-lowering agents given at the discretion of healthcare providers and according to recommendations of local guidelines.
-
b.
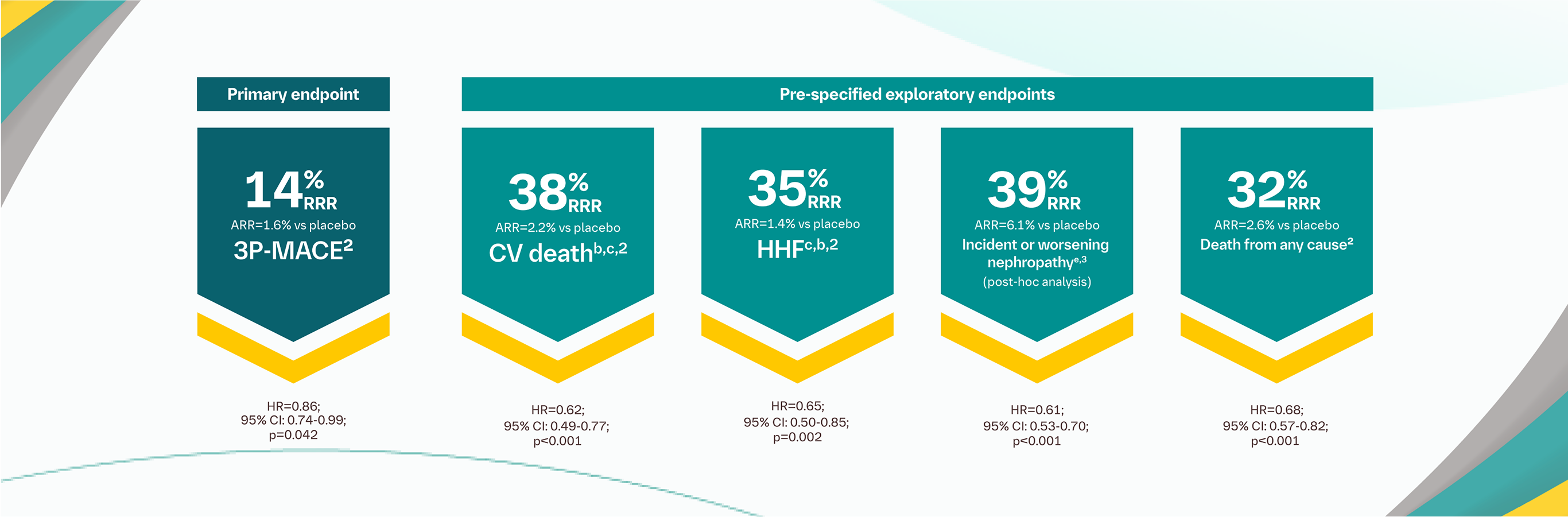
CV death was an exploratory endpoint.2
-
c.
Pooled data from 10 mg and 25 mg doses of JARDIANCE®; both doses showed a comparable reduction in the risk of CV death and HHF endpoints.2
-
d.
HHF was a exploratory endpoint.2
-
e.
Incident or worsening nephropathy is defined as progression to macroalbuminuria, doubling of serum creatinine, eGFR of ≤45 ml/min/1,73 m2; initiation of renal replacement therapy; death from renal disease. Incident or worsening nephropathy was a prespecified component of the exploratory microvascular outcome in the EMPA-REG OUTCOME® trial.3
-
f.
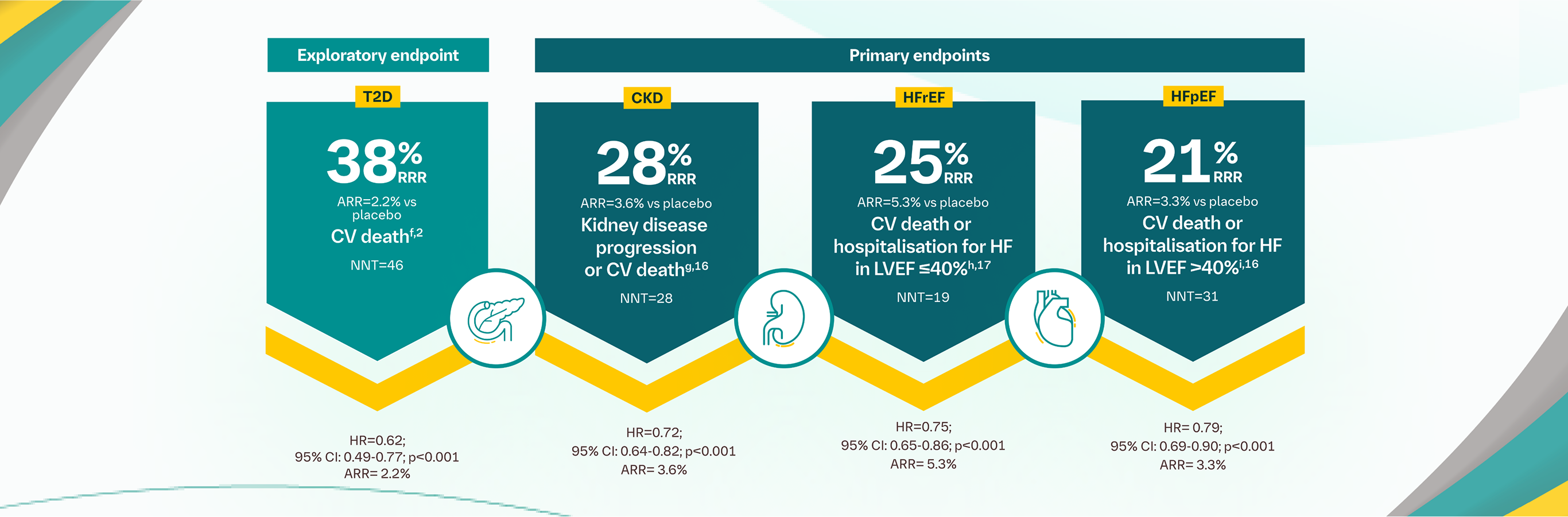
The primary composite outcome in the EMPA-REG OUTCOME® trial was 3-point MACE, composed of death from CV causes, nonfatal MI, or nonfatal stroke, as analysed in the pooled JARDIANCE® group vs the placebo group. Patients were adults with insufficiently controlled T2D and CAD, PAD, or a history of MI or stroke. The 14% RRR in 3-point MACE (HR=0.86; 95% CI: 0.74-0.99; p<0.001 for noninferiority; p=0.04 for superiority) was driven by a reduction in the risk of the exploratory endpoint CV death (HR=0.62; 95% CI: 0.49-0.77); there was no change in risk of nonfatal MI (HR=0.87; 95% CI: 0.70-1.09) or nonfatal stroke (HR=1.24; 95% CI: 0.92-1.67).2
-
g.
In the EMPA-KIDNEY® trial, a randomised, parallel-group, double-blind, placebo-controlled study of 6,609 patients with CKD, the efficacy and safety of JARDIANCE® 10 mg (n=3,304) were evaluated vs placebo (n=3,305). The primary endpoint in the EMPA-KIDNEY® trial was a composite of CV death or progression of kidney disease. Patients treated with JARDIANCE® experienced a 28% RRR in this endpoint (HR=0.72; 95% CI: 0.64-0.82; p<0.001).12
-
h.
In the EMPEROR-Reduced® trial, a randomised, double-blind, parallel-group, placebo-controlled study of 3,730 patients with HFrEF, the efficacy and safety of JARDIANCE® 10 mg (n=1,863) were evaluated vs placebo (n=1,867). Patients were adults with chronic HF (NYHA class II, III, or IV) and reduced ejection fraction (LVEF ≤40%). The primary endpoint in the EMPEROR-Reduced® trial was a composite of CV death or hospitalisation for HF, analysed as time to the first event. Patients treated with JARDIANCE® experienced a 25% RRR in this endpoint (HR=0.75; 95% CI: 0.65-0.86; p<0.001).13
-
i.
In the EMPEROR-Preserved® trial, a randomised, double-blind, parallel-group, placebo-controlled study of 5,988 patients with HFpEF, the efficacy and safety of JARDIANCE® 10 mg (n=2,997) were evaluated vs placebo (n=2,991). The primary endpoint in the EMPEROR-Preserved® trial was a composite of CV death or hospitalisation for HF, analysed as time to the first event. Patients treated with JARDIANCE® experienced a 21% RRR in this endpoint (HR=0.79; 95% CI: 0.69-0.90; p<0.001).14
Abbreviations
3P-MACE = 3 point-major adverse cardiovascular events; AE = adverse event; ARR = absolute risk reduction; CAD = coronary artery disease; CI = confidence interval; CKD = chronic kidney disease; CV = cardiovascular; CVD = cardiovascular disease; eGFR = estimated glomerular filtration rate; GTI = genital tract infection; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; HHF = hospitalisation for heart failure; HR = hazard ratio; LVEF = left ventricular ejection fraction; MI = myocardial infarction; NEJM = New England Journal of Medicine; NYHA = New York Heart Association; PAD = peripheral artery disease; UTI = urinary tract infection; vs = versus.
- JARDIANCE® (empagliflozin) UK Summary of Product Characteristics (SmPC). Available at: http://www.medicines.org.uk/emc/medicine/28973.
- Zinman B, Wanner C, Lachin JM, et al. EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. (EMPA-REG OUTCOME results and the publication’s Supplementary Appendix.)
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
- Wiviott SD, Raz, I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
- Cannon CP, Pratley R, Dagogo-Jack, et al. Cardiovascular outcomes with Ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383:1425-1435.
- Pratley RE, et al. American Diabetes Association (ADA) Virtual 88th Scientific Sessions. June 2020. Oral presentation.
- Wanner C, Inzucchi SE, Lachin JM, et al. EMPA-REG OUTCOME Investigators. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323-334.
- Tesfaye H, et al. Diabetes Obes Health 2025;27(6):3503-3508.
- Htoo PT, et al. Diabetologia 2024;67(7):1328-1342.
- Khan SE, ed. Standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S1-S291.
- Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022: a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753-2786.
- de Boer IH, Khunti K, Sadusky T, et al. Diabetes management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care. 2022;45(12):3075-3090.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024;105(4S):S117-S314.
- Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(17):e263-e421.
- McDonagh TA, Metra M, Adamo M, et al. ESC Scientific Document Group. 2023 Focused update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023;44(37):3627-3639.
- Herrington WG, Staplin N, Wanner C, et al. EMPA-KIDNEY Collaborative Group. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117-127. (EMPA-KIDNEY results and the publication’s Supplementary Appendix.)
- Packer M, Anker SD, Butler J, et al. EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413-1424. (EMPEROR-Reduced results and the publication’s Supplementary Appendix.)
- Anker SD, Butler J, Filippatos G, et al. EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451-1461. (EMPEROR-Preserved results and the publication’s Supplementary Appendix.)
PC-GB-111883 V2 | September 2025
The content on this website is in relation to adult patients.
Empagliflozin is not recommended in severe hepatic impairment, breastfeeding, Type 1 diabetes and is contraindicated in patients with hypersensitivity to the active ingredient or any of its excipients. Empagliflozin should be avoided in pregnancy.
Please consult the SmPC for full details regarding adverse events, monitoring requirements and interactions prior to prescribing JARDIANCE®.
- JARDIANCE® (empagliflozin) UK Summary of Product Characteristics (SmPC). Available at:
http://www.medicines.org.uk/emc/medicine/28973.
JARDIANCE® is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise
- as monotherapy when metformin is considered inappropriate due to intolerance
- in addition to other medicinal products for the treatment of diabetes1
JARDIANCE® is indicated in adults for the treatment of chronic kidney disease.1
JARDIANCE® is indicated in adults for the treatment of symptomatic chronic heart failure.1
PC-GB-109995 V4 | October 2025


