Key guidelines recommend SGLT2 inhibitors EARLY as a first line treatment alongside standard of care to offer
cardio-renal protection3-8
NICE NG28 recommends that adults with type 2 diabetes and CKD who are taking an ARB or ACE inhibitor (titrated to highest dose):
- are offered an SGLT2 inhibitor if ACR is >30mg/mmol
- are considered for an SGLT2 inhibitor if ACR is 3 — 30mg/mmol
Patients must meet the criteria in the marketing authorisation, including relevant eGFR thresholds:
- Endorses SGLT2 inhibitors early, first line with metformin, due to proven CV benefits to categories of adults with type 2 diabetes
- SGLT2 inhibitor is recommended as soon as metformin tolerability is confirmed
Please refer to the full guidelines for a complete list of recommendations before prescribing.
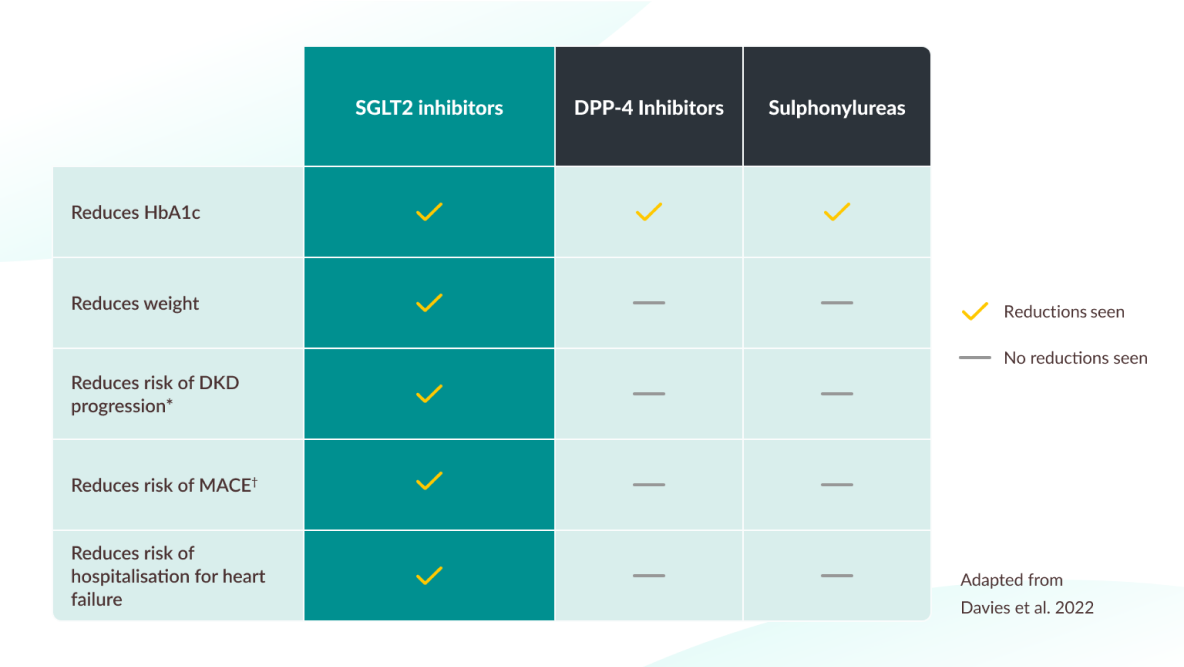
For patients on metformin consider adding an SGLT2i like JARDIANCE®6
A consensus report by the ADA and the EASD (2022).
The table has been adapted from ADA/EASD Consensus report 2022 and is not from head-to-head trials.
Please refer to the report for a complete list of recommendations. JARDIANCE® is not licensed for weight reduction. Licensed indications for SGLT2 inhibitors vary, please see individual SmPCs before prescribing.
-
*
Risk reduction seen with empagliflozin, canagliflozin and dapagliflozin
-
†
MACE is defined as one or more of the following: cardiovascular death, myocardial infarction, hospitalisation for heart failure and all cause mortality.
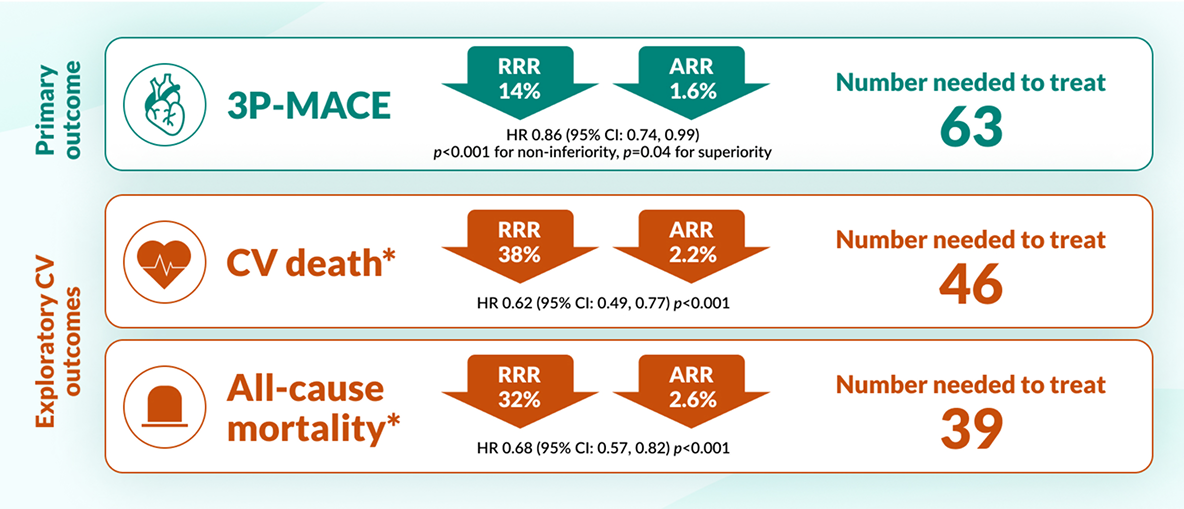
-
*
EMPA-REG Outcome® Trial: A randomised, double-blind, placebo-controlled cardiovascular outcomes trial (n=7020). Patients with T2D and CVD were randomised to receive JARDIANCE® (10mg or 25mg) (n=4687) or placebo (n=2333) once daily, on top of standard of care. Patients were adults with insufficiently controlled T2D and CAD, PAD, or a history of MI or stroke. Primary endpoint for JARDIANCE® (pooled figures for 10mg and 25mg dose vs placebo): 3-point MACE (time to first occurrence of CV death, non-fatal myocardial infarction or non-fatal stroke), 14% RRR (1.6% ARR) HR 0.86, 95% CI 0.74–0.99, p=0.04 for superiority. Pre-defined exploratory endpoint for JARDIANCE® (pooled figures for 10mg and 25mg dose vs placebo): CV death 38% RRR (2.2% ARR) (HR 0.62, 95% CI 0.49–0.77, p<0.001).9
Empagliflozin reduced mortality and risk of CV events vs placebo in adults with T2D and CVD9
CV death and all-cause mortality were predefined exploratory outcomes of the EMPA-REG Outcome® trial*.
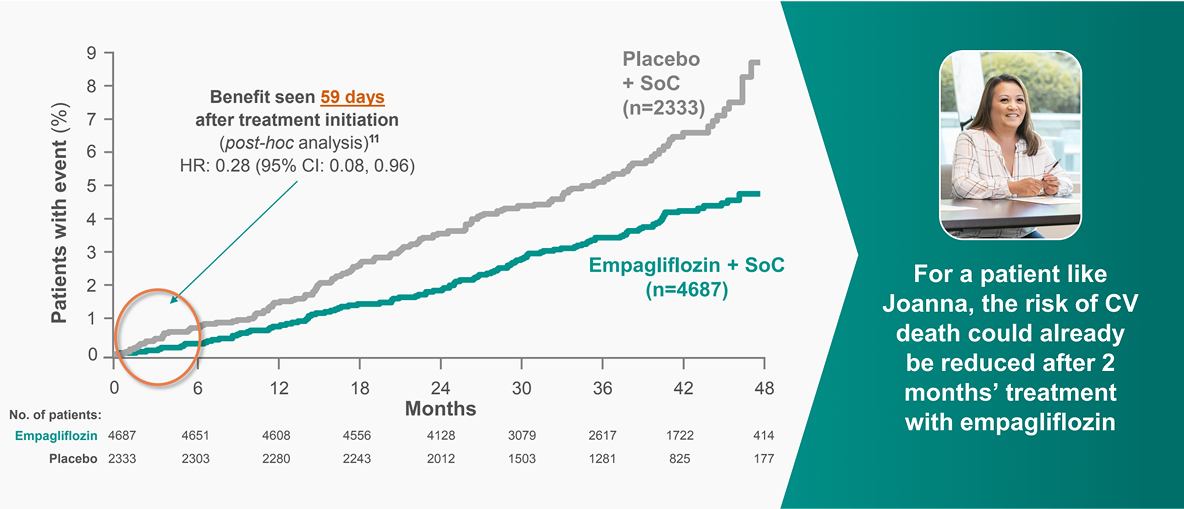
JARDIANCE® reduced the risk of CV death EARLY vs placebo9,11
EMPA-REG OUTCOME: pre-defined exploratory outcome and post-hoc analysis
CV death was a pre-defined adjudicated exploratory endpoint. Unless otherwise stated, pooled data for empagliflozin 10mg and 25mg are represented. The efficacy for preventing CV mortality has not been conclusively established in patients using empagliflozin concomitantly with DPP-4is or in black patients because the representation of these groups in the EMPA-REG OUTCOME trial was limited.9
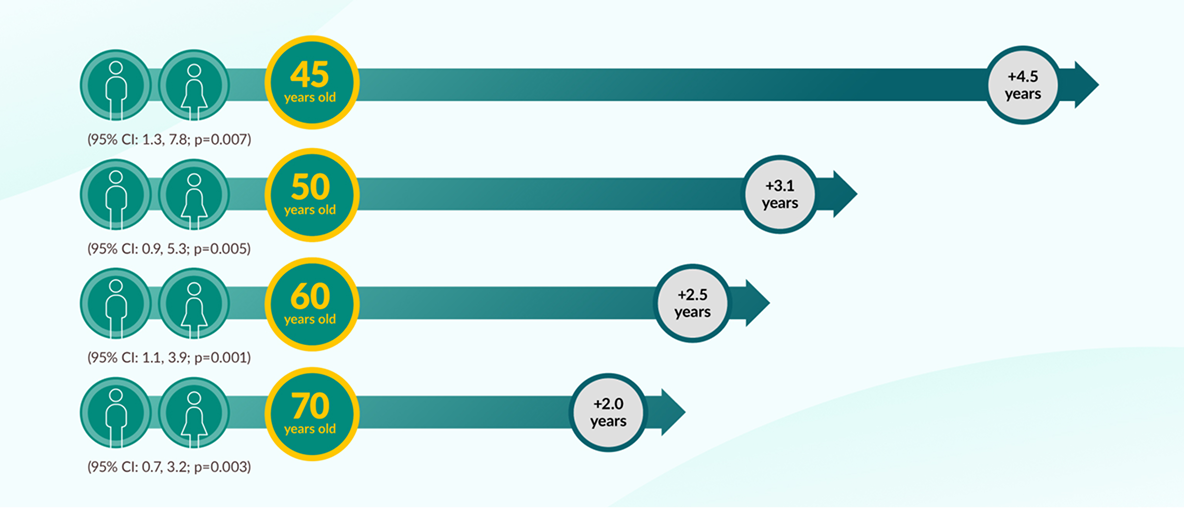
JARDIANCE® can add years of life to eligible patients with T2D and CVD10
Survival estimates vs placebo based on an actuarial analysis of data from the EMPA-REG OUTCOME® trial.
JARDIANCE® consistently increased estimated mean survival by 12 to 15% vs placebo. Mean patient age in the EMPA-REG OUTCOME® trial: 63 years. This is a survival estimate and may not apply to all patients.
Expand your learning
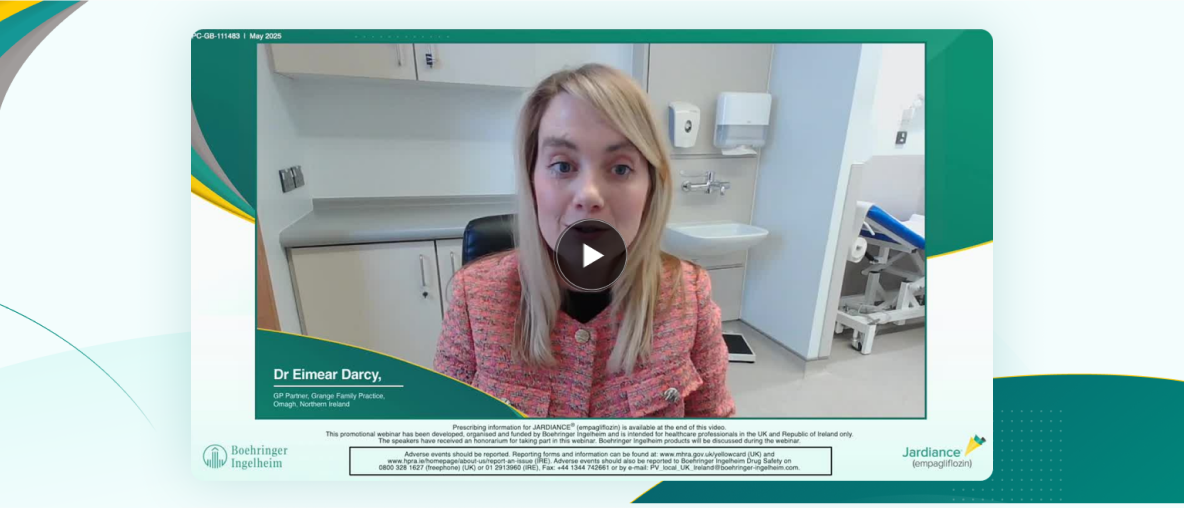
Full On-Demand Recording: Keeping it simple
Watch experts Sonia Willis and Dr Eimear Darcy explore optimising the management of type 2 diabetes and empowering patients to improve adherence.
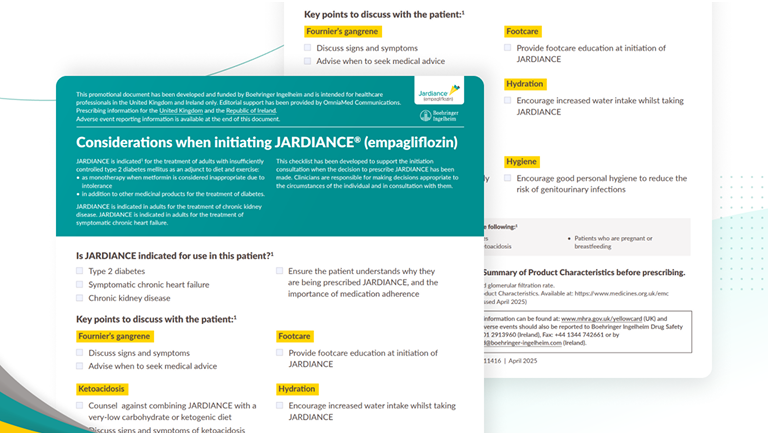
Checklist to support initiation of JARDIANCE®

JARDIANCE® patient booklet

Sick day rules: what should patients do with their medicines when they’re unwell?
Abbreviations
ADA: American Diabetes Association, ARR: absolute risk reduction, CI: confidence interval, CRM: cardio renal metabolism, CV: cardiovascular, CVD: cardiovascular disease, EASD: European Association for the Study of Diabetes, HR: hazard ratio, KDIGO: Kidney Disease: Improving Global Outcomes, MACE: major adverse cardiovascular events, MI: myocardial infarction, NICE: National Institute for Health and Care Excellence, PAD: peripheral artery disease, RRR: relative risk reduction, SGLT2: sodium-glucose co-transporter-2, SoC: standard of care, UKKA: UK Kidney Association.
- Liu R et al. J Diabetes Res. 2022;1531289.
- Ooi Y et al. Diabetes Metab J. 2024;48(2):196-207.
- National Institute for Health and Care Excellence (NICE) 2023. Empagliflozin for treating chronic heart failure with preserved or mildly reduced ejection fraction. Technology Appraisal Guidance TA929. Available from: https://www.nice.org.uk/guidance/ta929.
- National Institute for Health and Care Excellence (NICE) (2022). Type 2 diabetes in adults: management. NG28. Available at: www.nice.org.uk/guidance/ng28.
- National Institute for Health and Care Excellence (NICE) 2015. Empagliflozin in combination therapy for treating type 2 diabetes. Technology Appraisal Guidance TA336. Available from: https://www.nice.org.uk/guidance/ta336.
- Davies MJ et al. Diabetes Care 2022;45(11):2753–2786.
- UKKA treatment of CKD recommendations 2023. Available at: https://guidelines.ukkidney.org/summary-of-recommendations/.
- Kidney Disease: Improving Global outcomes (KDIGO) CKD Work Group. Kidney Int. 2024;105(4S):S117-S314.
- Zinman B, et al. N Engl J Med. 2015;373(22):2117–2128.
- Claggett B, et al. Circulation. 2018;138:1599–1601.
- Verma S et al. ESC Heart Fail. 2021;8(4):2603–2607.
PC-GB-111713 V2 | September 2025
The content on this website is in relation to adult patients.
Empagliflozin is not recommended in severe hepatic impairment, breastfeeding, Type 1 diabetes and is contraindicated in patients with hypersensitivity to the active ingredient or any of its excipients. Empagliflozin should be avoided in pregnancy.
Please consult the SmPC for full details regarding adverse events, monitoring requirements and interactions prior to prescribing JARDIANCE®.
- JARDIANCE® (empagliflozin) UK Summary of Product Characteristics (SmPC). Available at:
http://www.medicines.org.uk/emc/medicine/28973.
JARDIANCE® is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise
- as monotherapy when metformin is considered inappropriate due to intolerance
- in addition to other medicinal products for the treatment of diabetes1
JARDIANCE® is indicated in adults for the treatment of chronic kidney disease.1
JARDIANCE® is indicated in adults for the treatment of symptomatic chronic heart failure.1
PC-GB-109995 V4 | October 2025