Why early intervention matters
On average, a 55-year-old female with cardiovascular disease (MI and stroke) and diabetes dies 17 years earlier than a female of the same age with neither condition.1
By acting early, you can make a meaningful impact—adding years to life and life to years. Learn how JARDIANCE® can support you.
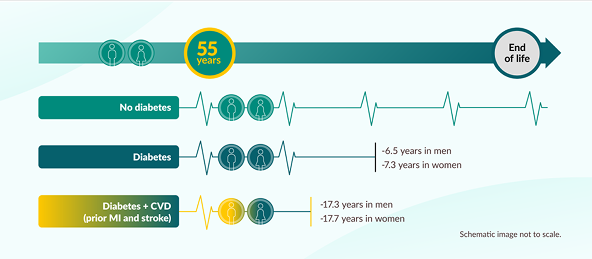
Cumulative survival was estimated by applying calculated age-specific HRs for mortality to contemporary US age-specific death rates. Data based on the analysis of Emerging Risk Factors Collaboration (ERFC) results from 689,300 participants compared with UK Biobank, a prospective cohort study of 499,808 participants. Years of life lost is according to age 55 at baseline compared with people with neither diabetes nor CVD.
Key guidelines recommend SGLT2 inhibitors EARLY as a first line treatment alongside standard of care to offer cardio-renal protection.
Watch Dr Jarvis and Dr Javaid discuss NICE guidance updates (NG28) and the positioning of SGLT2 inhibitors.
-
*
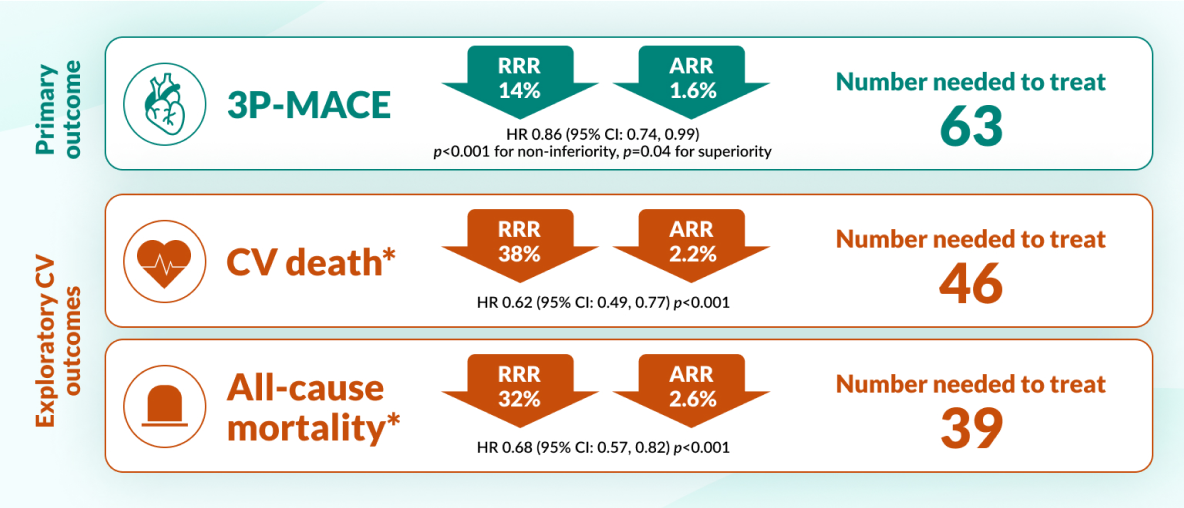
EMPA-REG Outcome® Trial: A randomised, double-blind, placebo-controlled cardiovascular outcomes trial (n=7020). Patients with T2D and CVD were randomised to receive JARDIANCE® (10mg or 25mg) (n=4687) or placebo (n= 2333) once daily, on top of standard of care. Patients were adults with insufficiently controlled T2D and CAD, PAD, or a history of MI or stroke. Primary endpoint for JARDIANCE® (pooled figures for 10mg and 25mg dose vs placebo): 3-point MACE (time to first occurrence of CV death, non-fatal myocardial infarction or non-fatal stroke), 14% RRR (1.6% ARR) HR 0.86, 95% CI 0.74–0.99, p=0.04 for superiority. Pre-defined exploratory endpoint for JARDIANCE® (pooled figures for 10mg and 25mg dose vs placebo): CV death 38% RRR (2.2% ARR) (HR 0.62, 95% CI 0.49–0.77, p<0.001).8
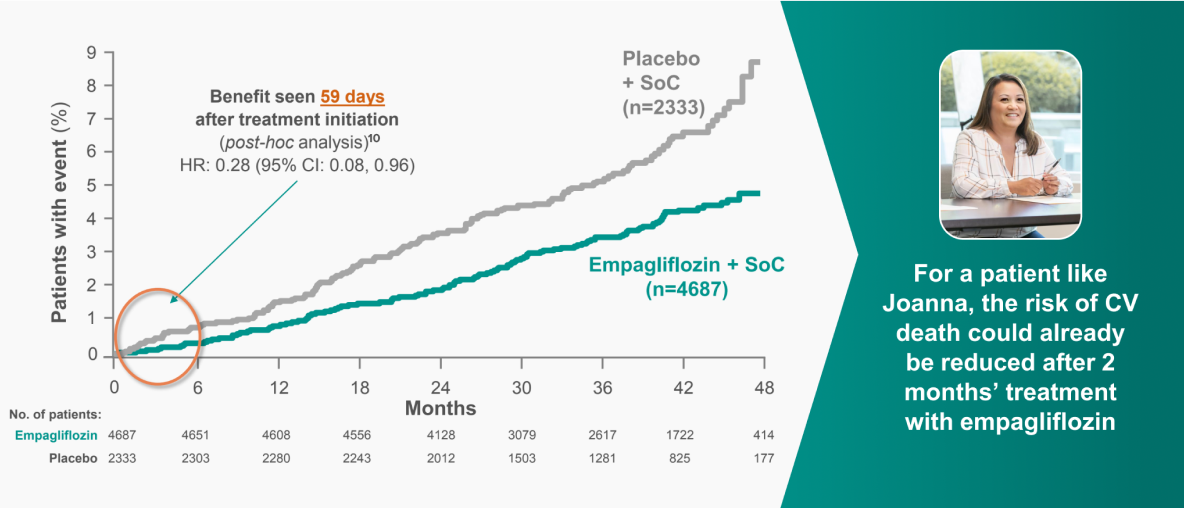
Empagliflozin reduced mortality and risk of CV events vs placebo in adults with T2D and CVD8
CV death and all-cause mortality were predefined exploratory outcomes of the EMPA-REG Outcome® trial*.
JARDIANCE® reduced the risk of CV death EARLY vs placebo8
EMPA-REG OUTCOME: pre-defined exploratory outcome and post-hoc analysis
CV death was a pre-defined adjudicated exploratory endpoint. Unless otherwise stated, pooled data for empagliflozin 10 mg and 25 mg are represented. The efficacy for preventing CV mortality has not been conclusively established in patients using empagliflozin concomitantly with DPP-4is or in black patients because the representation of these groups in the EMPA-REG OUTCOME trial was limited.8
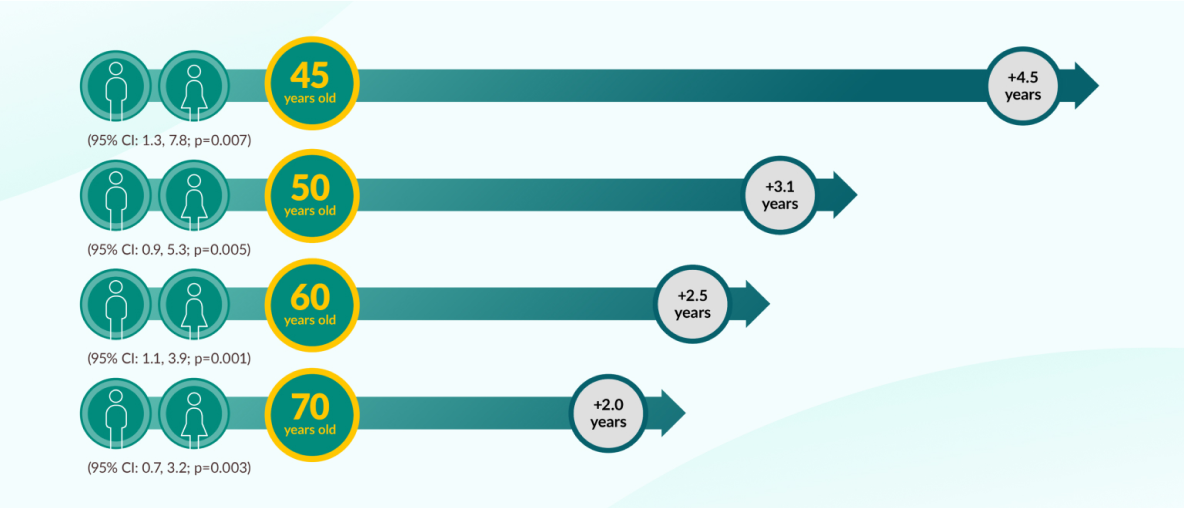
JARDIANCE® can add years of life to eligible patients with T2D and CVD9
Survival estimates vs placebo based on an actuarial analysis of data from the EMPA-REG OUTCOME® trial.
JARDIANCE® consistently increased estimated mean survival by 12 to 15% vs placebo. Mean patient age in the EMPA-REG OUTCOME® trial: 63 years. This is a survival estimate and may not apply to all patients.
Latest resource
Webinar - 5 minute highlight - BRIDGING THE GAP
Watch Yassir Javaid discussing the importance of SGLT2is for patients with type 2 diabetes and high cardiovascular risk.
Prefer to watch the full recording? Click here to view the full webinar recording.

JARDIANCE® patient booklets

JARDIANCE® Initiation & Management guide for T2D, CHF and CKD

JARDIANCE® patient booklet
Abbreviations
ADA: American Diabetes Association, ARR: absolute risk reduction, CI: confidence interval, CRM: cardio renal metabolism, CV: cardiovascular, CVD: cardiovascular disease, EASD: European Association for the Study of Diabetes, HR: hazard ratio, KDIGO: Kidney Disease: Improving Global Outcomes, MACE: major adverse cardiovascular events, MI: myocardial infarction, NICE: National Institute for Health and Care Excellence, PAD: peripheral artery disease, RRR: relative risk reduction, SGLT2: sodium-glucose co-transporter-2, SoC: standard of care, UKKA: UK Kidney Association.
- The Emerging Risk Factors Collaboration. JAMA. 2015;314(1):52-60. (and the publication’s Supplementary Appendix).
- National Institute for Health and Care Excellence (NICE) 2023. Empagliflozin for treating chronic heart failure with preserved or mildly reduced ejection fraction. Technology Appraisal Guidance TA929. Available from: https://www.nice.org.uk/guidance/ta929.
- National Institute for Health and Care Excellence (NICE) (2022). Type 2 diabetes in adults: management. NG28. Available at: www.nice.org.uk/guidance/ng28.
- National Institute for Health and Care Excellence (NICE) 2015. Empagliflozin in combination therapy for treating type 2 diabetes. Technology Appraisal Guidance TA336. Available from: https://www.nice.org.uk/guidance/ta336.
- Davies MJ et al. Diabetes Care 2022;45(11):2753–2786.
- UKKA treatment of CKD recommendations 2023. Available at: https://guidelines.ukkidney.org/summary-of-recommendations/.
- Kidney Disease: Improving Global outcomes (KDIGO) CKD Work Group. Kidney Int. 2024;105(4S):S117-S314.
- Zinman B, et al. N Engl J Med. 2015;373(22):2117–2128.
- Claggett B, et al. Circulation. 2018;138:1599–1601.
- Verma S et al. ESC Heart Fail. 2021;8(4):2603–2607.
- Neal B, et al. N Engl J Med. 2017;377:644–657.
- Wiviott SD, et al. N Engl J Med. 2019;380:347–357.
- Cannon CP, et al. N Engl J Med. 2020;383:1425–1435.
- Pratley RE, et al. American Diabetes Association (ADA) Virtual 88th Scientific Sessions. June 2020. Oral presentation.
PC-GB-111503 V4 | November 2025
The content on this website is in relation to adult patients.
Empagliflozin is not recommended in severe hepatic impairment, breastfeeding, Type 1 diabetes and is contraindicated in patients with hypersensitivity to the active ingredient or any of its excipients. Empagliflozin should be avoided in pregnancy.
Please consult the SmPC for full details regarding adverse events, monitoring requirements and interactions prior to prescribing JARDIANCE®.
- JARDIANCE® (empagliflozin) UK Summary of Product Characteristics (SmPC). Available at:
http://www.medicines.org.uk/emc/medicine/28973.
JARDIANCE® is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise
- as monotherapy when metformin is considered inappropriate due to intolerance
- in addition to other medicinal products for the treatment of diabetes1
JARDIANCE® is indicated in adults for the treatment of chronic kidney disease.1
JARDIANCE® is indicated in adults for the treatment of symptomatic chronic heart failure.1
PC-GB-109995 V4 | October 2025