SPIOLTO® Respimat® (tiotropium + olodaterol) delivers statistically significant improvements in health-related quality of life (as indicated by a reduction in SGRQ total score) vs placebo and SPIRIVA® Respimat (tiotropium)1-3
OTEMTO: Patients with a ≥4.0 unit improvement in SGRQ score vs baseline (measured at 12 weeks)1
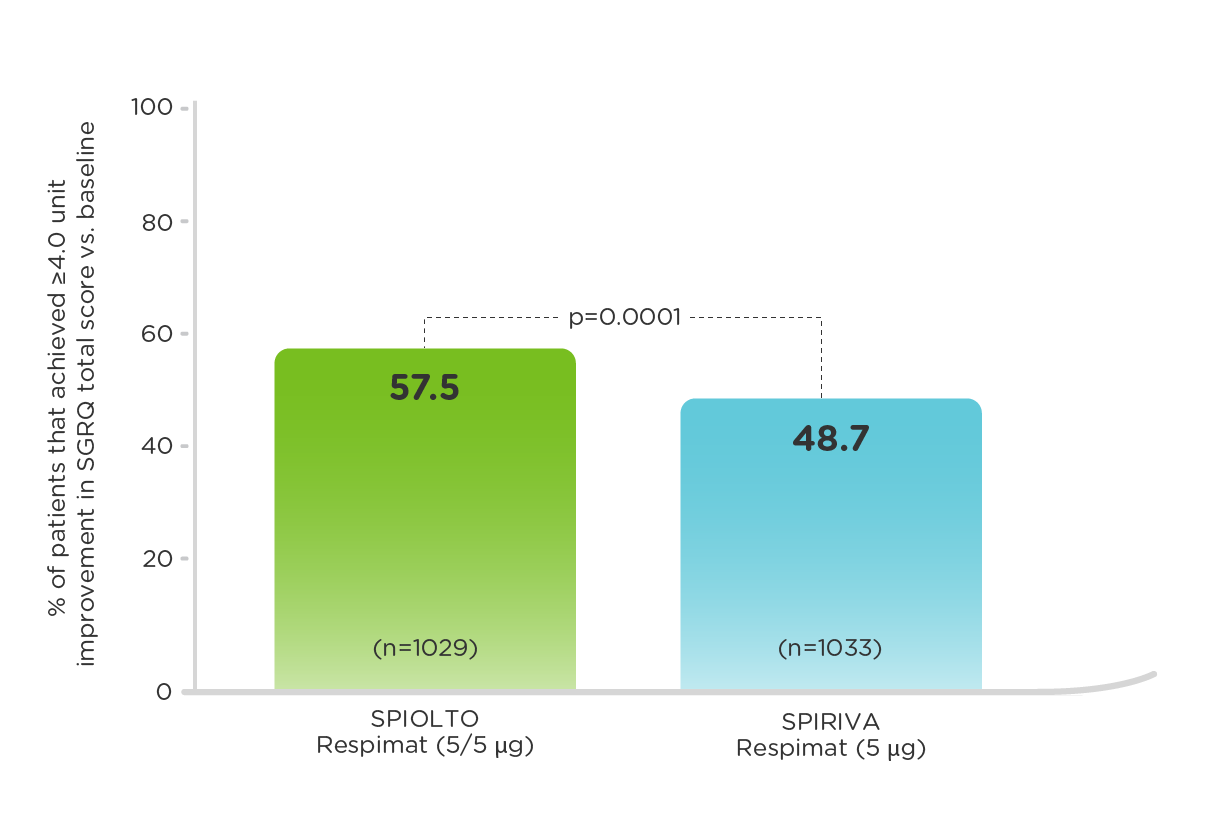
SPIOLTO Respimat improved SGRQ total score by 4.7 units vs placebo (p<0.0001), and by 2.1 units vs SPIRIVA Respimat (p<0.01).2
TONADO: Patients with a ≥4.0 unit improvement in SGRQ score vs baseline (measured at 24 weeks)1,3
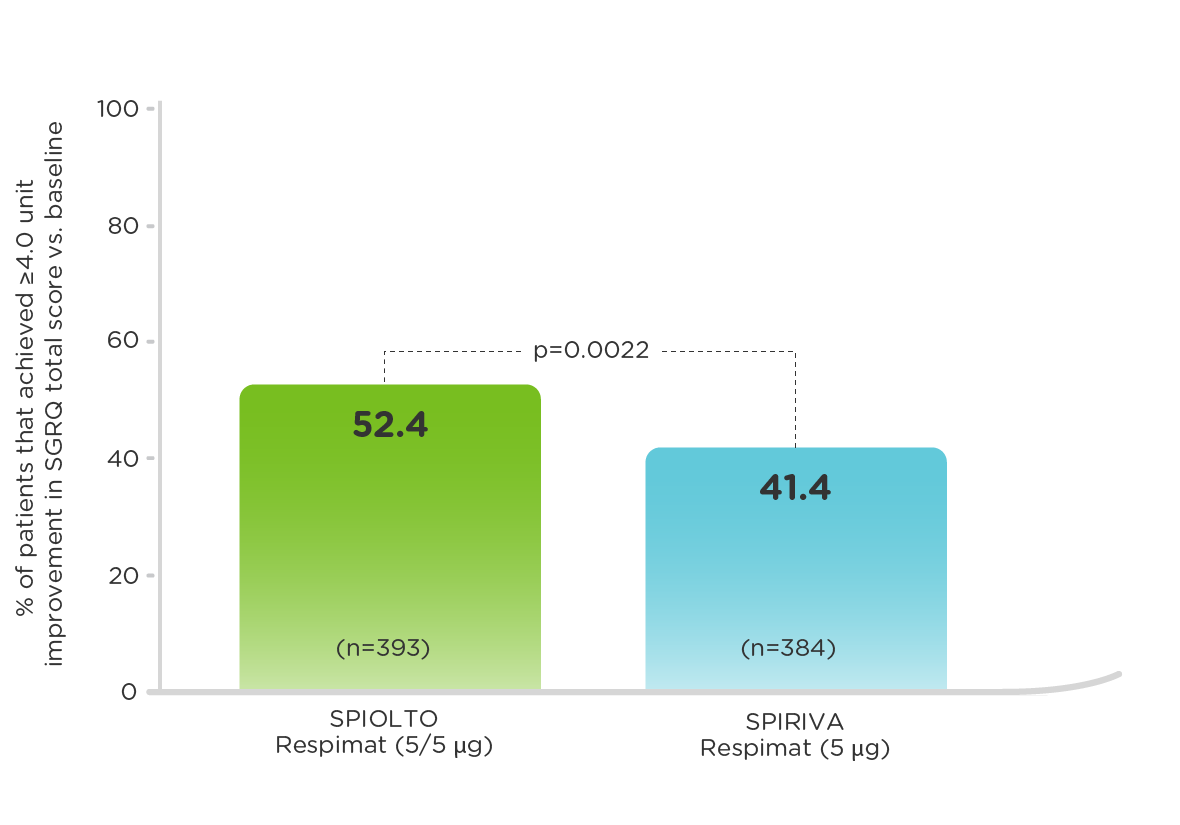
SPIOLTO Respimat improved SGRQ total score by 1.23 units vs SPIRIVA Respimat (p=0.025).1,3
SGRQ, St George’s Respiratory Questionnaire.
Study design
TONADO: Two replicate, randomised, double-blind, active-controlled, phase III trials comparing SPIOLTO Respimat with SPIRIVA Respimat (tiotropium) and olodaterol Respimat over 52 weeks in 5,162 adult patients with moderate to very severe COPD. The primary endpoints were lung function measured as FEV1 AUC0–3h and trough FEV1 response in each individual trial, and SGRQ total score (combined analysis of both trials) at 24 weeks.
OTEMTO: Two replicate, randomised, double-blind, placebo-controlled, phase IIIb trials comparing SPIOLTO Respimat with SPIRIVA Respimat (tiotropium) and placebo Respimat over 12 weeks in 1,621 adult patients with moderate to severe COPD. The primary endpoints were SGRQ total score, and lung function measured as FEV1 AUC0–3h and trough FEV1 response in each individual trial.
References: 1. SPIOLTO Respimat (tiotropium + olodaterol) Summary of Product Characteristics; 2. Singh D, et al. Respir Med. 2015;109:1312‒1319; 3. Buhl R, et al. Eur Respir J. 2015;45:969‒979.