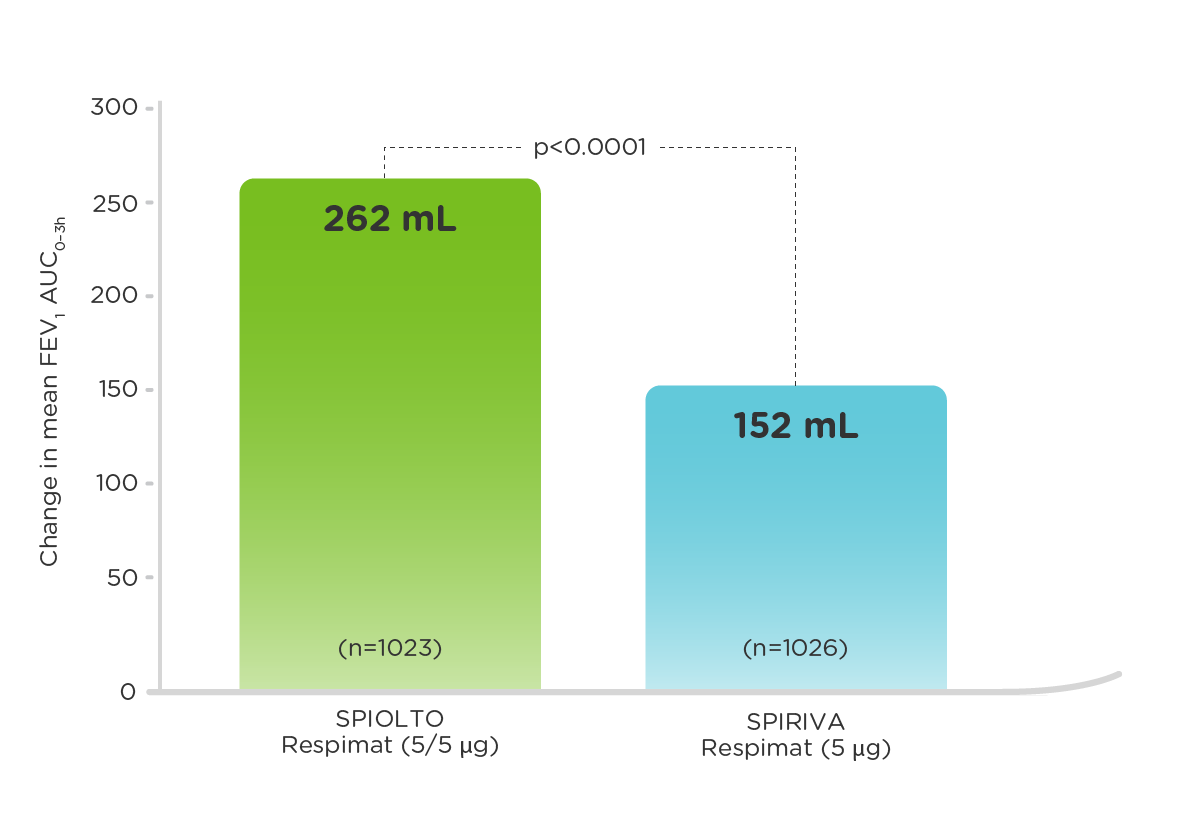
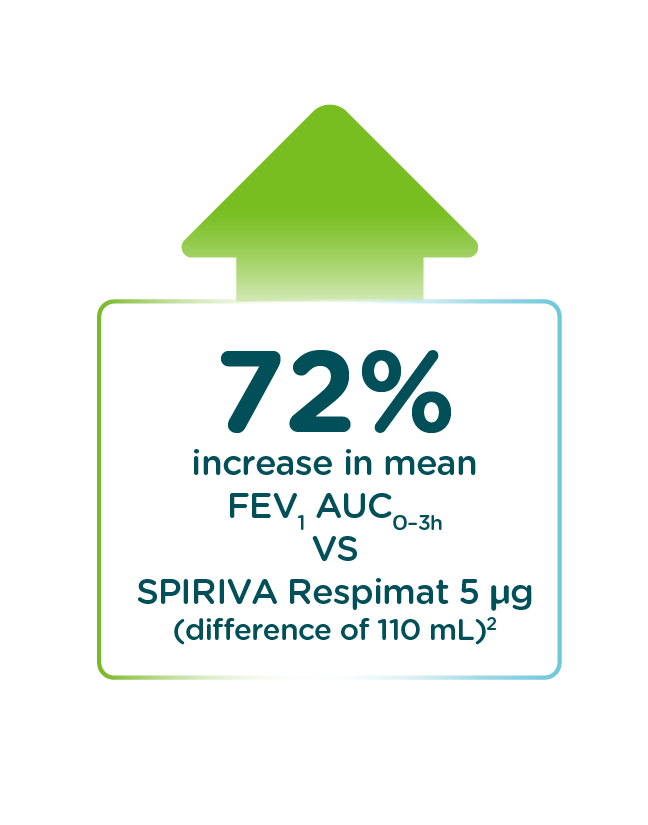
SPIOLTO® Respimat® (tiotropium + olodaterol) delivers statistically significant improvements in lung function (measured by FEV1 AUC0-3h and trough FEV1 response) vs SPIRIVA® Respimat (tiotropium)1,2
Mean change in FEV1 AUC0–3h (measured at 24 weeks)2
| 
|
SPIOLTO Respimat significantly improved mean trough FEV1 (difference of 60mL; p<0.0001) vs SPIRIVA Respimat.1
AUC0–3h, area under the curve 0–3 hours; FEV1, forced expiratory volume in one second.
Study design
TONADO: Two replicate, randomised, double-blind, active-controlled, phase III trials comparing SPIOLTO Respimat with SPIRIVA Respimat (tiotropium) and olodaterol Respimat over 52 weeks in 5,162 adult patients with moderate to very severe COPD. The primary endpoints were lung function measured as FEV1 AUC0–3h and trough FEV1 response in each individual trial, and SGRQ total score (combined analysis of both trials) at 24 weeks.
References: 1. Buhl R, et al. Eur Respir J. 2015;45:969‒979 and supplementary materials; 2. Boehringer Ingelheim Data on File TOL 14-05(b).