SPIOLTO® Respimat® (tiotropium + olodaterol) delivers statistically significant improvements in breathlessness vs SPIRIVA® Respimat (tiotropium)1,2
Change in breathlessness in the OTEMTO and TONADO trials, measured by TDI focal score*1,2
OTEMTO (measured at 12 weeks)1
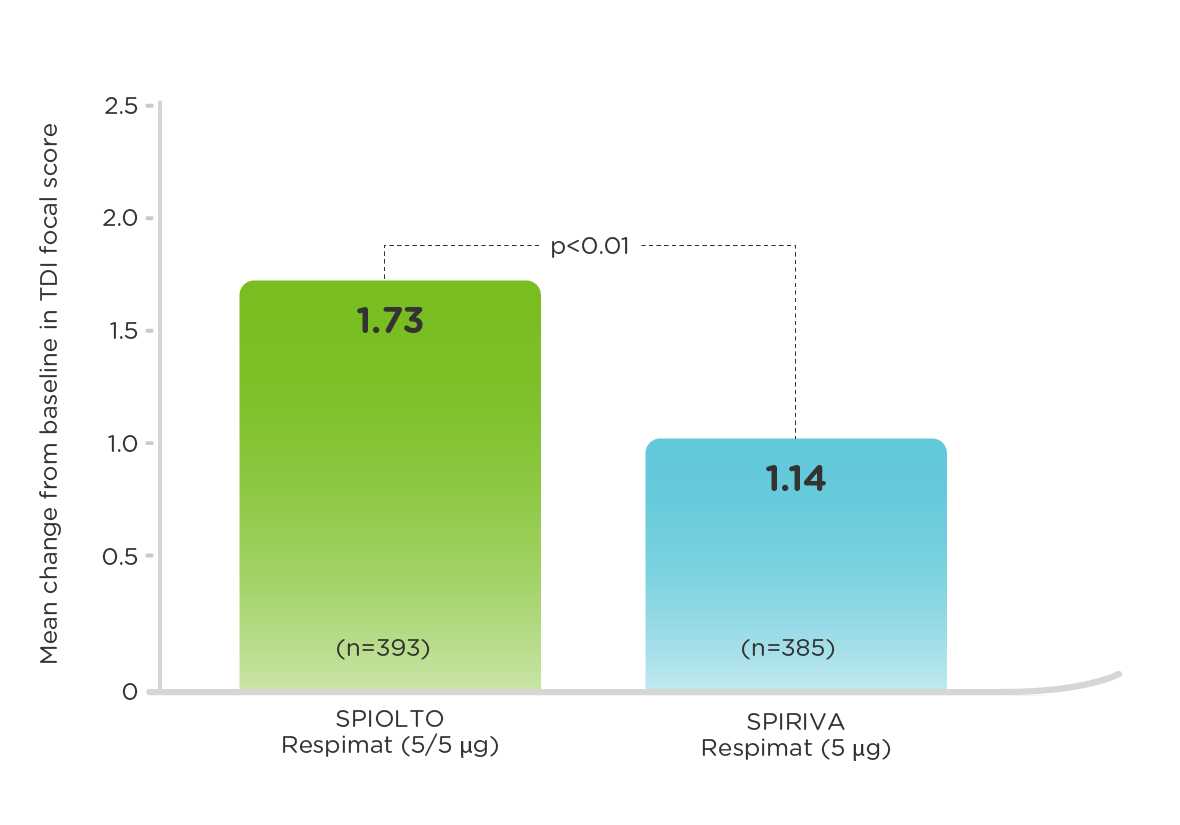
More patients treated with SPIOLTO Respimat had a clinically meaningful improvement in TDI focal score (MCID, defined as a value of at least 1 unit) compared to SPIRIVA Respimat (54% vs. 41%, p<0.001).3
TONADO (measured at 24 weeks)2
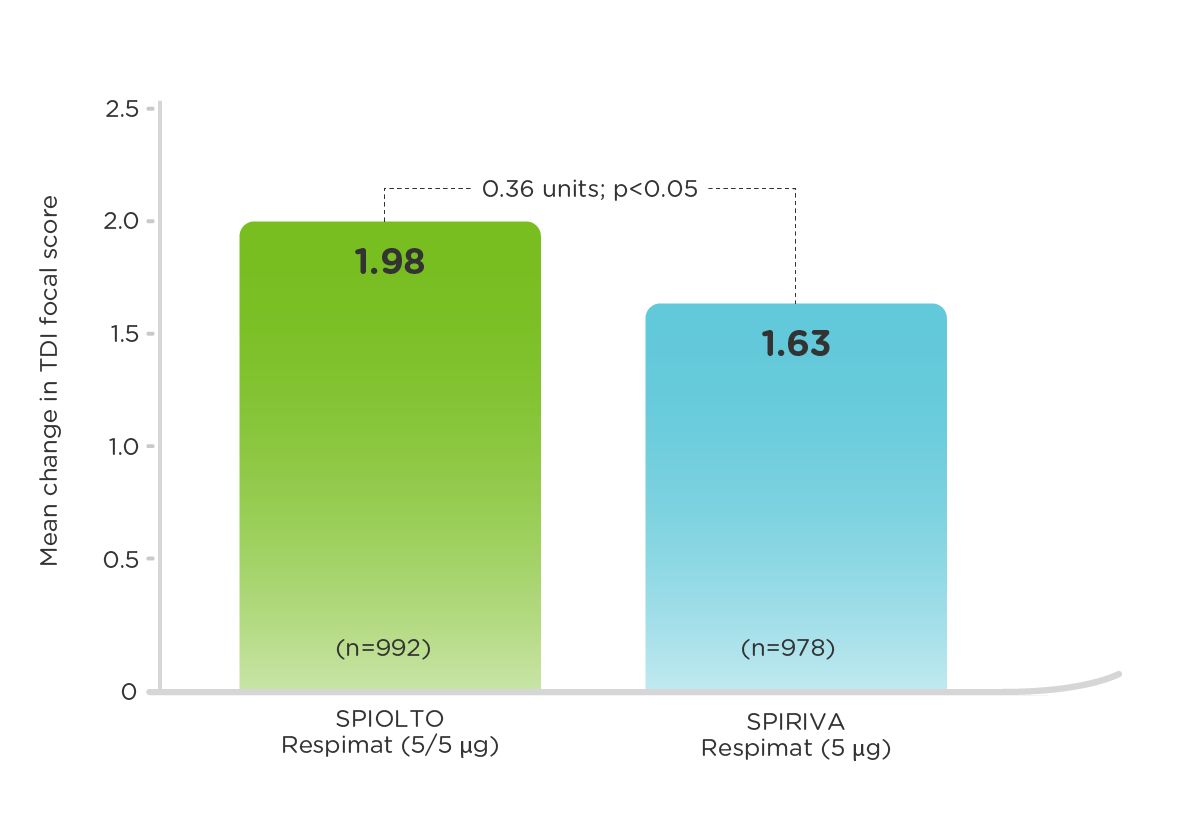
More patients treated with SPIOLTO Respimat had a clinically meaningful improvement in TDI focal score (MCID, defined as a value of at least 1 unit) compared to SPIRIVA Respimat (54.9% vs. 50.6%, p=0.0546).3,4
-
*
Breathlessness, as measured by Transitional Dyspnoea Index (TDI) focal score, was a secondary endpoint in the OTEMTO (measured at 12 weeks) and TONADO (measured at 24 weeks) trials. An increase in TDI score indicates an improvement in breathlessness. A 1-unit change in the TDI focal score is considered clinically important.1,2
MCID, minimum clinically important difference; TDI, transition dyspnoea index.
Study design
TONADO: Two replicate, randomised, double-blind, active-controlled, phase III trials comparing SPIOLTO Respimat with SPIRIVA Respimat (tiotropium) and olodaterol Respimat over 52 weeks in 5,162 adult patients with moderate to very severe COPD. The primary endpoints were lung function measured as FEV1 AUC0–3h and trough FEV1 response in each individual trial, and SGRQ total score (combined analysis of both trials) at 24 weeks.
OTEMTO: Two replicate, randomised, double-blind, placebo-controlled, phase IIIb trials comparing SPIOLTO Respimat with SPIRIVA Respimat (tiotropium) and placebo Respimat over 12 weeks in 1,621 adult patients with moderate to severe COPD. The primary endpoints were SGRQ total score, and lung function measured as FEV1 AUC0–3h and trough FEV1 response in each individual trial.
References: 1. Singh D, et al. Respir Med. 2015;109:1312‒1319; 2. Buhl R, et al. Eur Resp J. 2015;45:969‒979 and supplementary materials; 3. Ferguson GT, et al. NPJ Prim Care Respir Med. 2017;27:7; 4. SPIOLTO Respimat (tiotropium + olodaterol) Summary of Product Characteristics.