SPIRIVA® Respimat® significantly delayed the time to first asthma worsening* by 134 days vs placebo Respimat in adults with severe asthma.1
Time to first asthma worsening (pooled data at 48 weeks) was a secondary endpoint.
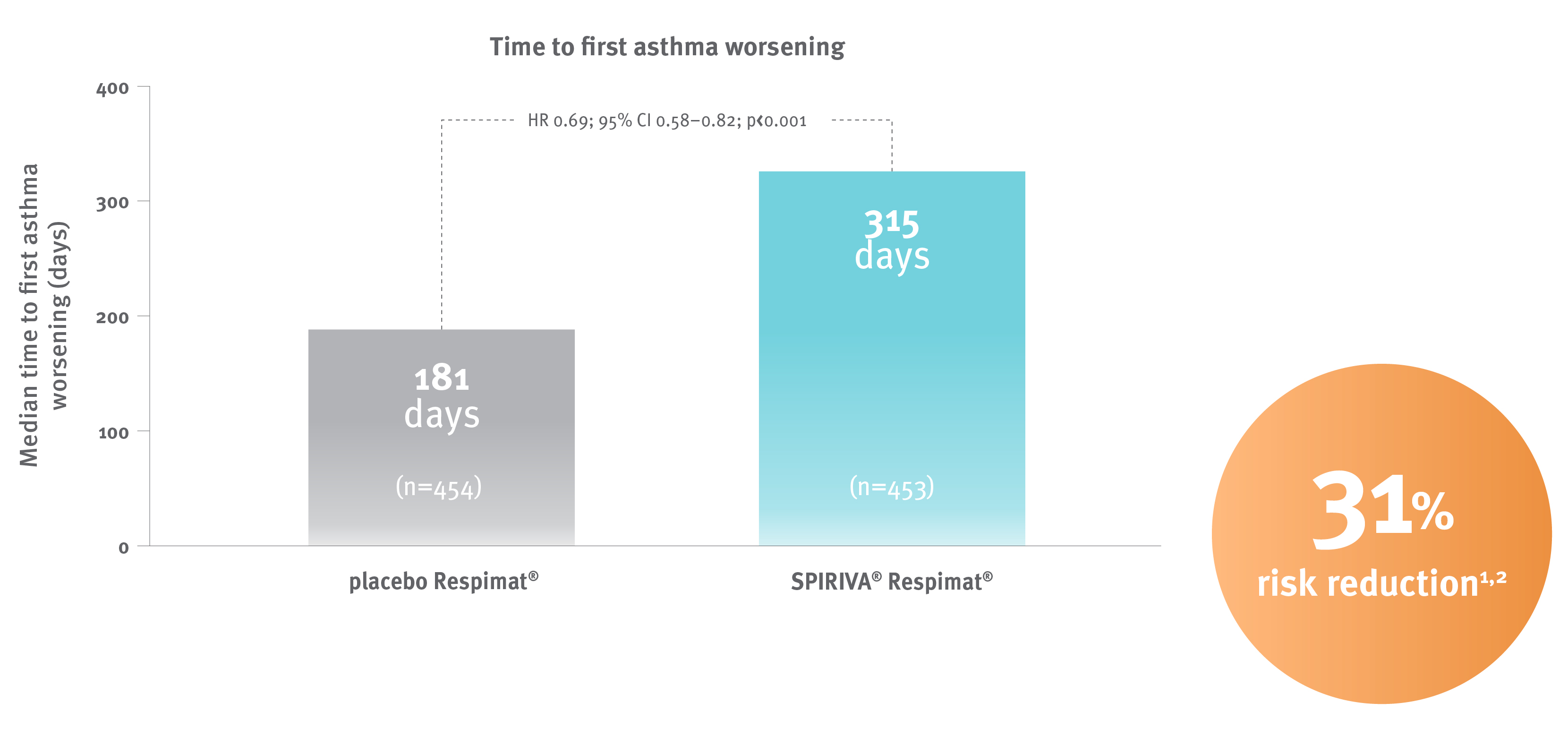
In the tiotropium group, 226 of 453 patients (49.9%) had at least 1 episode of asthma worsening, as compared with 287 of 454 (63.2%) in the placebo group.1
* Asthma worsening was defined as either a progressive increase in symptoms (as compared with usual day-to-day asthma symptoms) or a decline of 30% or more in the best morning PEF from the mean screening morning PEF for 2 or more consecutive days.1
Adapted from Kerstjens HA et al.1
PrimoTinA Study Design
Two replicate randomised controlled trials (PrimoTinA-Asthma 1 and PrimoTinA-Asthma 2) compared SPIRIVA Respimat with placebo Respimat over 48 weeks as add-on controller therapy on top of usual care in adult patients with severe asthma who were symptomatic on maintenance treatment of at least ICS (≥800 μg budesonide/day or equivalent) plus LABA, had a post-bronchodilator FEV1 of ≤80% of the predicted value and a history of ≥1 severe exacerbation in the previous year. In both the SPIRIVA Respimat and placebo Respimat groups, ICS and LABA maintenance therapy was continued. Other asthma medications were allowed if the doses remained stable for ≥4 weeks before study entry and for the duration of the trial. The co-primary endpoints were lung function measured as peak FEV1(0-3h) and trough FEV1 response at week 24, and time to first severe exacerbation (pooled data) over 48 weeks.1
Abbreviations
ARR, absolute risk reduction; CI, confidence interval; HR, hazard ratio; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; PEF, peak expiratory flow.
Reference: 1. Kerstjens HA et al. N Engl J Med 2012;367:1198–207.